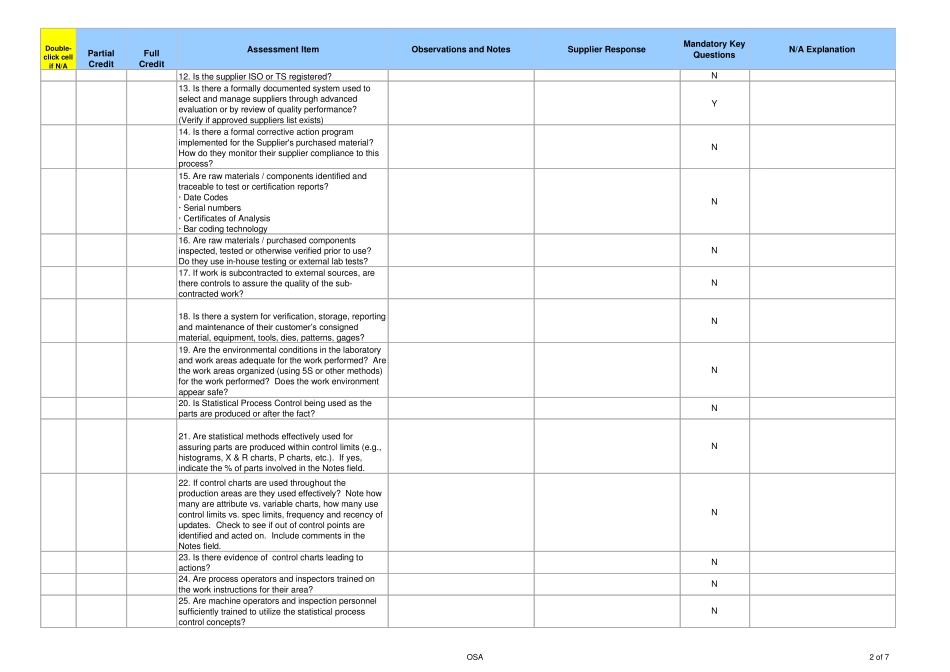
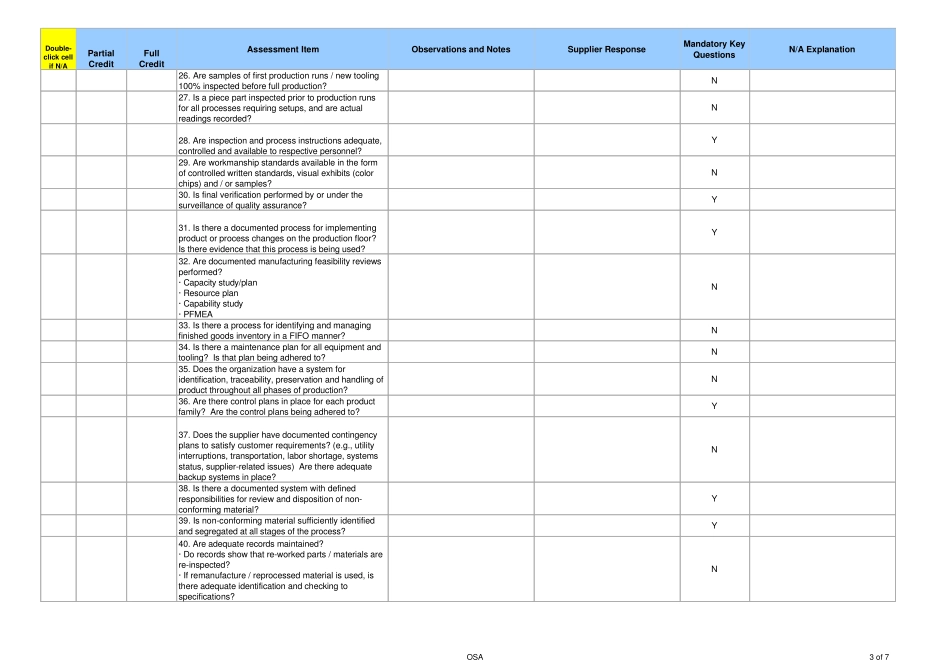
Double-click cellif N/APartialCreditFullCreditAssessment ItemObservations and NotesSupplier ResponseMandatory KeyQuestionsN/A Explanation1. Is there a published Quality Policy and is itcommunicated to all personnel (e.g., Quality Manual)?N2. Is there an organization chart with responsibilitiesassigned? Is there an independent set of resourcesassigned to assuring the quality of the productprovided to IR?N3. Are the quality control systems adequatelydocumented?· Work Instructions· Standard Operating Procedures· Quality Objectives· External DocumentsY4. Do systems and processes focus on defectprevention in all areas?N5. Has training been provided for each job function oroperation according to requirements? Can a trainingmatrix and current training records be provided?Y6. Is there a documented internal audit process and dothey perform scheduled product and system audits toassure compliance with defined quality requirements?· Audit schedule· Adherence to scheduleN7. Are systems and audit results periodically reviewedby management?· Management Reviews frequency· Agenda and meeting minutes from the lastManagement ReviewN8. Is there a Corrective Action system and does it havea means to track/measure performance of thesystem?· Number of CA’s are processed in a year· Number of CA’s that are past dueY9. Do systems and audit deficiency corrective actionshave a due date and are they closed on time?N10. Is there a Preventative Action system and does ithave a means to track/measure performance of thesystem?· Number of PA’s are processed in a year· Number of PA’s that are past dueN11. Is there a process established for the control ofrecords and system documentation?· Policy for control of quality records· Retention policy or mat...