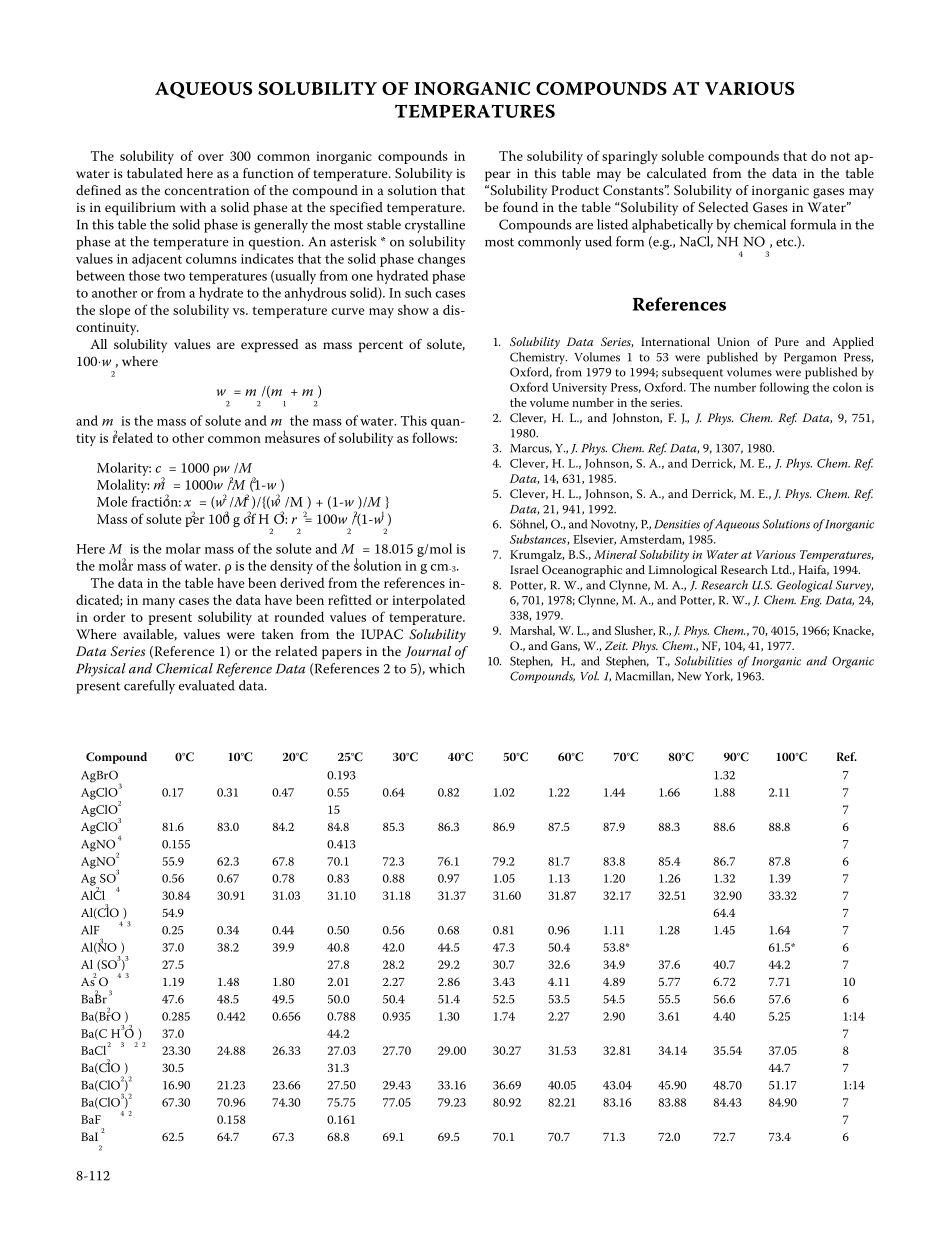
AQUEOUS SOLUBILITY OF INORGANIC COMPOUNDS AT VARIOUS TEMPERATURES The solubility of over 300 common inorganic compounds in water is tabulated here as a function of temperature. Solubility is defined as the concentration of the compound in a solution that is in equilibrium with a solid phase at the specified temperature. In this table the solid phase is generally the most stable crystalline phase at the temperature in question. An asterisk * on solubility values in adjacent columns indicates that the solid phase changes between those two temperatures (usually from one hydrated phase to another or from a hydrate to the anhydrous solid). In such cases the slope of the solubility vs. temperature curve may show a dis-continuity.All solubility values are expressed as mass percent of solute, 100⋅w2, where w2 = m2/(m1 + m2)and m2 is the mass of solute and m1 the mass of water. This quan-tity is related to other common measures of solubility as follows:Molarity: c2 = 1000 ρw2/M2Molality: m2 = 1000w2/M2(1-w2)Mole fraction: x2 = (w2/M2)/{(w2/M2) + (1-w2)/M1}Mass of solute per 100 g of H2O: r2 = 100w2/(1-w2)Here M2 is the molar mass of the solute and M1 = 18.015 g/mol is the molar mass of water. ρ is the density of the solution in g cm-3.The data in the table have been derived from the references in-dicated; in many cases the data have been refitted or interpolated in order to present solubility at rounded values of temperature. Where available, values were taken from the IUPAC Solubility Data Series (Reference 1) or the related papers in the Journal of Physical and Chemical Reference Data (References 2 to 5), which present carefully evaluated data.The solubility of sparingly soluble compounds tha...