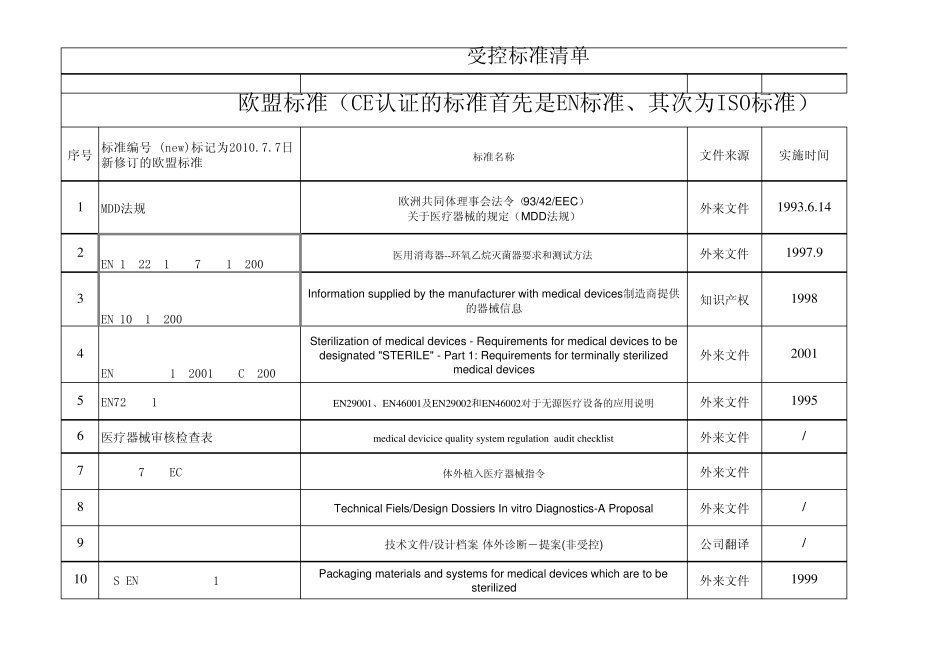
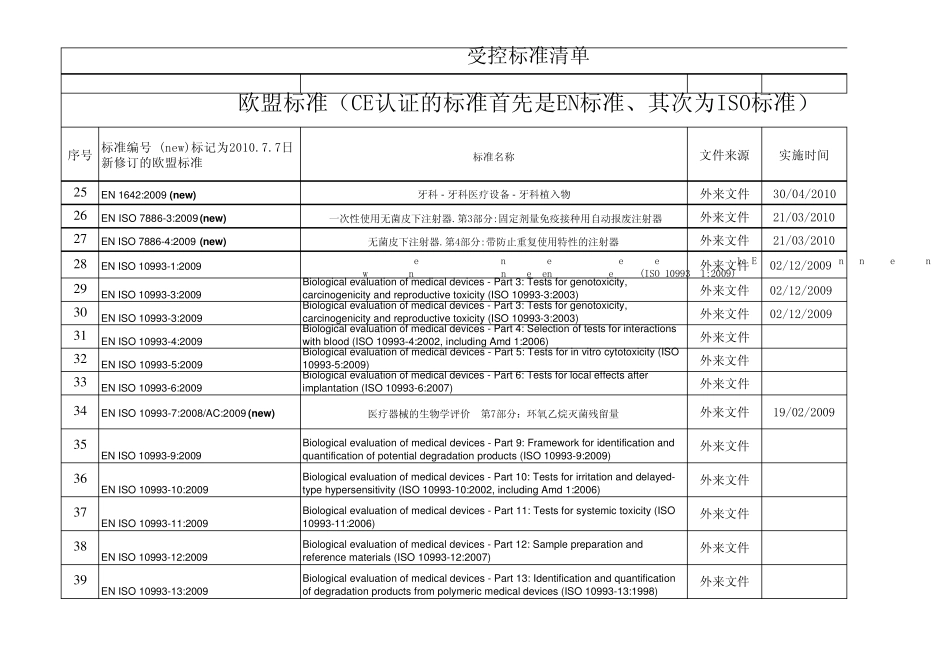
序号标准编号 (new)标记为2010.7.7日新修订的欧盟标准标准名称文件来源实施时间1MDD法规欧洲共同体理事会法令(93/42/EEC)关于医疗器械的规定(MDD法规)外来文件1993.6.142EN 1422:1997+A1:2009医用消毒器--环氧乙烷灭菌器要求和测试方法外来文件1997.93EN 1041:2008Information supplied by the manufacturer with medical devices制造商提供的器械信息知识产权 19984EN 556-1:2001/AC:2006Sterilization of medical devices - Requirements for medical devices to bedesignated "STERILE" - Part 1: Requirements for terminally sterilizedmedical devices外来文件 20015EN724:1995EN29001、EN46001及EN29002和EN46002对于无源医疗设备的应用说明外来文件 19956医疗器械审核检查表medical dev icice qu ality sy stem regu lation au dit checklist外来文件/798-79-EC体外植入医疗器械指令外来文件8ivd-dd-tfTechnical Fiels/Design Dossiers In vitro Diagnostics-A Proposal外来文件/9ivd-dd-tf技术文件/设计档案 体外诊断-提案(非受控)公司翻译/10BS EN868-5:1999Packaging materials and systems for medical devices which are to besterilized外来文件 1999受控标准清单欧盟标准(CE认证的标准首先是EN标准、其次为ISO标准)序号标准编号 (new)标记为2010.7.7日新修订的欧盟标准标准名称文件来源实施时间受控标准清单欧盟标准(CE认证的标准首先是EN标准、其次为ISO标准)11MEDDEV.2.12-1:2009医疗器械警戒系统指南外来文件2008.1.112EN 980:2008Symbols for use in the labelling of medical devices外来文件2010.5.3113BS EN 61010-2-040-2005Safety requirements for electrical equipment for measurement, control and laboratory usePart 2-040: Particular requirements for sterilizers and washer-disinfectors used to treatmedical materials购买2005.71497/23/EC(PED) 压力设备 Pressure equipmen购买1997.5.2915Certification of OEM Devices OEM设备的认证购买16GHTF SG3 N15R8风险管理原则和活动在质量管理体系中的实施购买2005.5.2017软件确认基本原则:工业界及FDA人员指南购买2002.1.1118EN 1041:2008Information supplied by the manufacturer with medical devices制造商提供的器械信息外...