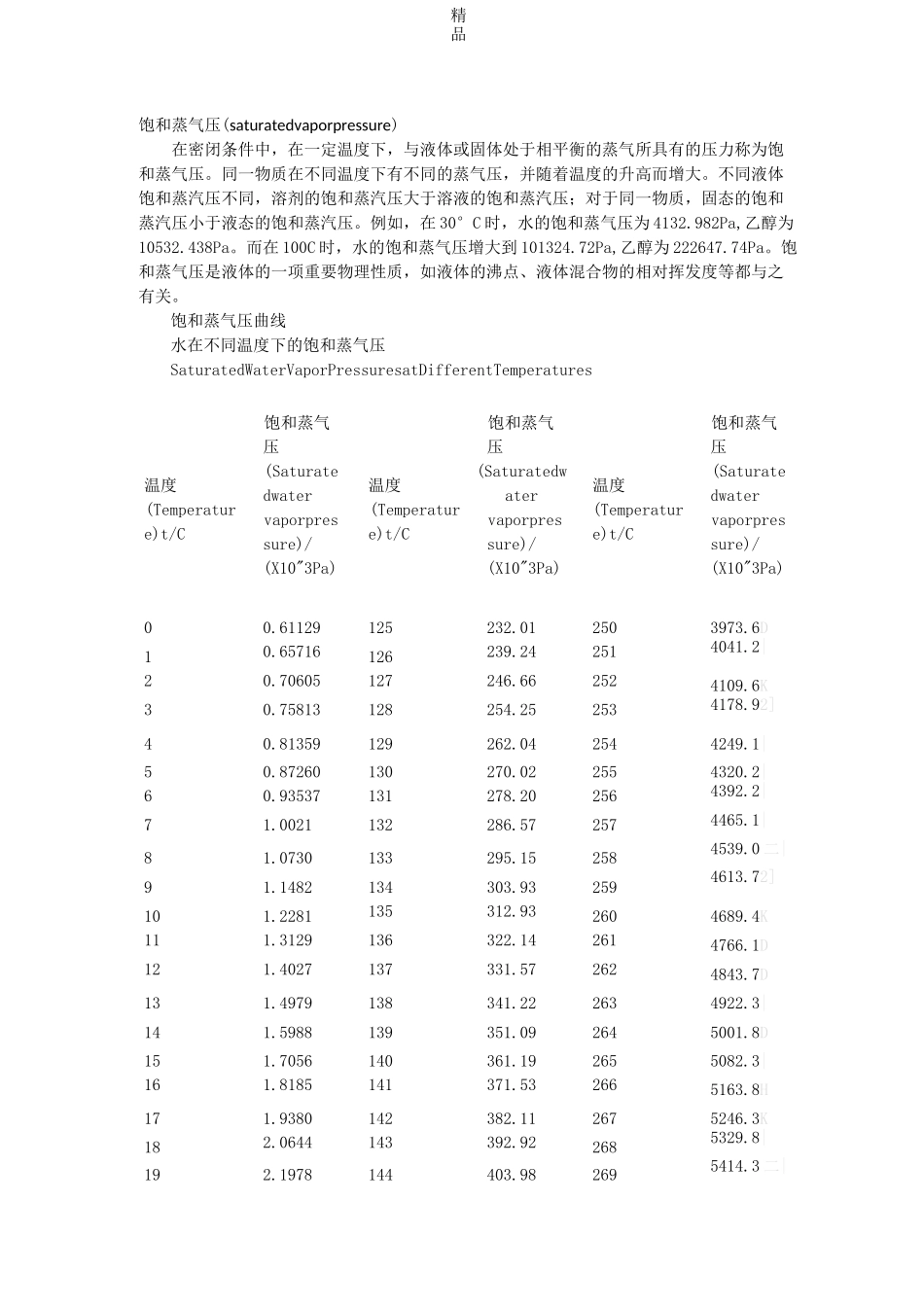
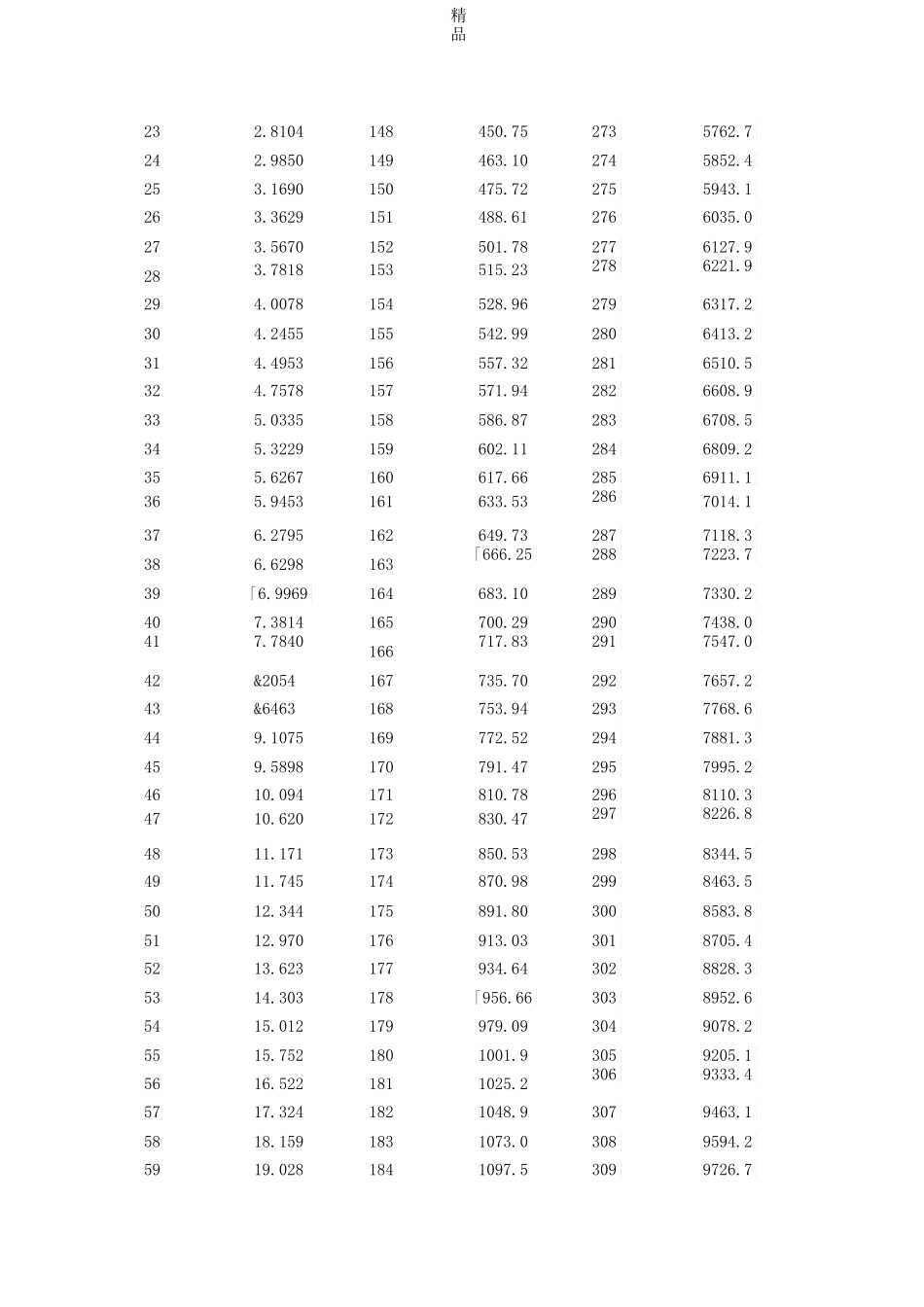
精品饱和蒸气压(saturatedvaporpressure)在密闭条件中,在一定温度下,与液体或固体处于相平衡的蒸气所具有的压力称为饱和蒸气压。同一物质在不同温度下有不同的蒸气压,并随着温度的升高而增大。不同液体饱和蒸汽压不同,溶剂的饱和蒸汽压大于溶液的饱和蒸汽压;对于同一物质,固态的饱和蒸汽压小于液态的饱和蒸汽压。例如,在 30°C 时,水的饱和蒸气压为 4132.982Pa,乙醇为10532.438Pa。而在 100C 时,水的饱和蒸气压增大到 101324.72Pa,乙醇为 222647.74Pa。饱和蒸气压是液体的一项重要物理性质,如液体的沸点、液体混合物的相对挥发度等都与之有关。饱和蒸气压曲线水在不同温度下的饱和蒸气压SaturatedWaterVaporPressuresatDifferentTemperatures温度(Temperature)t/C饱和蒸气压(Saturatedwatervaporpressure)/(X10"3Pa)温度(Temperature)t/C饱和蒸气压(Saturatedwatervaporpressure)/(X10"3Pa)温度(Temperature)t/C饱和蒸气压(Saturatedwatervaporpressure)/(X10"3Pa)00.61129125232.012503973.6D10.65716126239.242514041.2|20.70605127246.662524109.6K30.75813128254.252534178.92]40.81359129262.042544249.1|50.87260130270.022554320.2|60.93537131278.202564392.2|71.0021132286.572574465.1|81.0730133295.152584539.0 二|91.1482134303.932594613.72]101.2281135312.932604689.4K111.3129136322.142614766.1D121.4027137331.572624843.7D131.4979138341.222634922.3|141.5988139351.092645001.8D151.7056140361.192655082.3|161.8185141371.532665163.8H171.9380142382.112675246.3K182.0644143392.922685329.8|192.1978144403.982695414.3 二|精品202.3388145415.29270〔5499.9|212.4877146426.852715586.4|222.6447147438.672725674.0K精品232.8104148450.75273|5762.7|242.9850149463.10274|5852.4|253.1690150475.72275|5943.1|263.3629151488.61276|6035.0|273.5670152501.78277|6127.9|283.7818153515.23278|6221.9|294.0078154528.96279|6317.2|304.2455155542.99280|6413.2|314.4953156557.32281|6510.5|324.7578157571.94282|6608.9|335.0335158586.87283|6708.5|345.3229159602.11284|6809.2|355.6267160617.66285|6911.1|365.9453161633.53286|7014.1376.2795162649.73287|7118.3|386.6298163「666.25|288|7223.7|39「6.9969|164683.10289|7330.2|407.3814165700.29290|7438.0|417.7840166717.83291|7547.0|42...