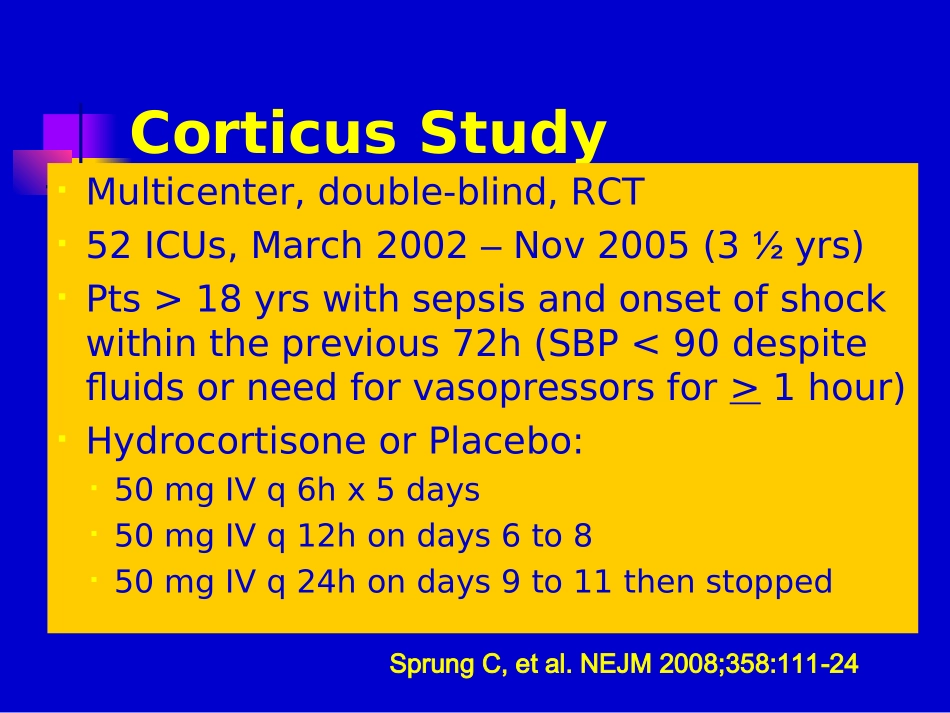
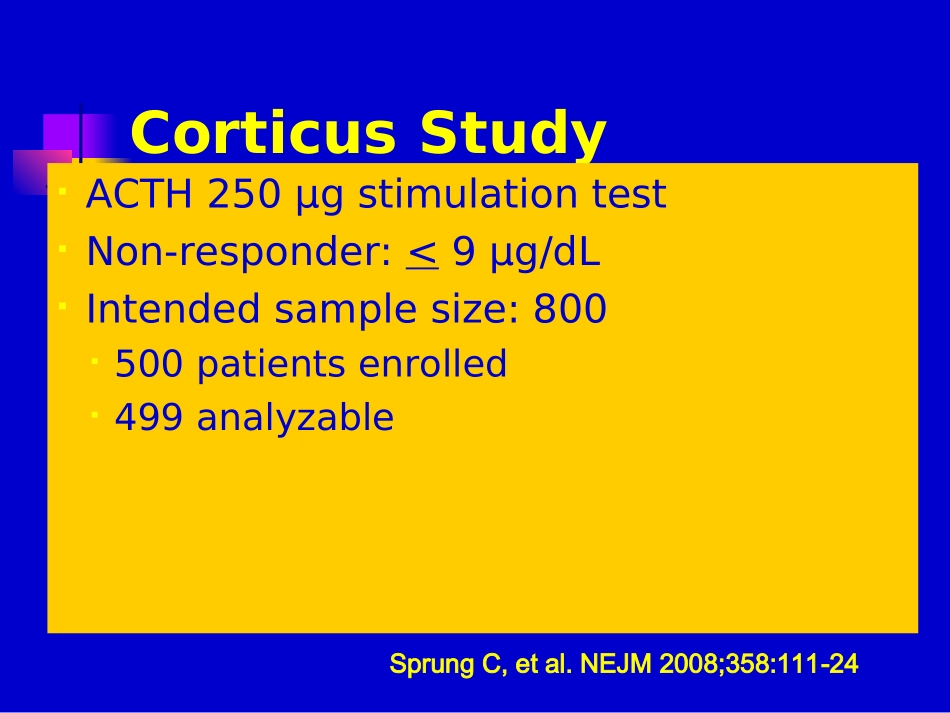
全身炎症反应综合症与脓毒血症(下)XXXX医院CorticusStudyMulticenter,double-blind,RCT52ICUs,March2002–Nov2005(3½yrs)Pts>18yrswithsepsisandonsetofshockwithintheprevious72h(SBP<90despitefluidsorneedforvasopressorsfor>1hour)HydrocortisoneorPlacebo:50mgIVq6hx5days50mgIVq12hondays6to850mgIVq24hondays9to11thenstoppedSprungC,etal.NEJM2008;358:111-24CorticusStudyACTH250μgstimulationtestNon-responder:<9μg/dLIntendedsamplesize:800500patientsenrolled499analyzableSprungC,etal.NEJM2008;358:111-24ACTHStimulationTest*Steroidsn=252Placebon-248Nonresponders125(49.8%)108(43.5%)Responders118(47%)136(54%)Unknown8(3.2%)4(1.6%)SprungC,etal.NEJM2008;358:111-24*Etomidatewasusedin51of251ptsinHCgroup(20.3%)andin45of248ptsinplacebogroup(18.1%)beforestudyentryplacebosteroidsurvival00.250.500.751.00051015202530day28daymortality:allpatientsp=0.51p=0.69placebosteroidsurvival00.250.500.751.00051015202530day28daymortality:non-respondersp=1.00placebosteroidsurvival00.250.500.751.00051015202530day28daymortality:responderssepticshock00.250.500.751.00051015202530dayplacebosteroidTimetoshockreversal:non-responderssepticshock00.250.500.751.00051015202530dayplacebosteroidTimetoshockreversal:respondersCORTICUS:ConclusionsHydrocortisoneRX•Didnotdecreasemortality•Deceasedtimetoshockreversal•Wasassoc.withanincreasedincidenceof:Superinfections,includingnewepisodesofsepsisorsepticshockHyperglycemiaHypernatremiaSprungC,etal.NEJM2008;358:111-24Annanevs.CORTICUSAnnaneCORTICUSTreatmentstart<8hofshock<72hofshockFludrocortisoneYesNoSteroidtaperNoYesMoremedicalptsYesNoMoresurgptsNoYesMoreintra-abd’lsourceofinfxnNoYesPlacebomortality61%31%#Nonresponders77%44%ClinicalPracticeGuidelinesfortheDiagnosisandManagementofCorticosteroidInsufficiencyinCriticalIllness:RecommendationsfromanInternationalTaskForceMarikPE,PastoresSM,AnnaneD,MeduriGU,SprungC,etal.CritCareMed(underreview)ConsensusStatementAtthistime,CIRCIisbestdiagnosedbyadeltacortisol(following250µgcosyntropin)of<9µg/dlorarandomcortisolof<10µg/dlFreecortisolhasadvantagesovertotalcortisolbutnotwidelyavailableTheACTHstimtestshouldnotbeusedtoidentifythesubsetofadultptswithsepticshockwhoshouldreceivehydrocortisone(2B)MarikPE,PastoresSM,AnnaneD,MeduriGU,SprungC,etal.CritCareMed2008(underreview)AdrenalTaskForceConsensusPanelTreatmentandDurationTreatmentregimens:•100mghydrocortisoneIVq8h•100/200mgbolusofhydrocortisonethen10mg/h•50mghydrocortisoneIVq6hFulldosehydrocortisonetreatmentshouldbecontinuedfor5-7daysbeforetaperingassumingthereisnorecurrenceofsignsofsepsisorshock(2C)MarikPE,PastoresSM,AnnaneD,MeduriGU,SprungC,etal.CritCareMed2008(underreview)ConsensusStatementPatientswithsepticshockshouldnotreceivedexamethasoneifhydrocortisoneisavailable(2B)Fludrocortisoneisoptionalifhydrocortisoneisused(2C)Dosesofcorticosteroidscomparableto>300mgofhydrocortisonedailynotbeusedinsepticshock(1A)MarikPE,PastoresSM,AnnaneD,MeduriGU,SprungC,etal.CritCareMed2008(underreview);SCC2008Update2008Drotrecoginalfa(activated)Drotrecoginalfa(activated)(rhAPC)inSevere(rhAPC)inSevereSepsisSepsisRecombinantHumanActivatedProteinCRecommendedinadultptswithsepsis-inducedorgandysfunctionassociatedwithahighriskofdeath(APACHEII>25)ormultipleorganfailureandwithnocontraindicationsrelatedtobleedingGrade2BAdultpatientswithseveresepsisandlowriskofdeath(AP...