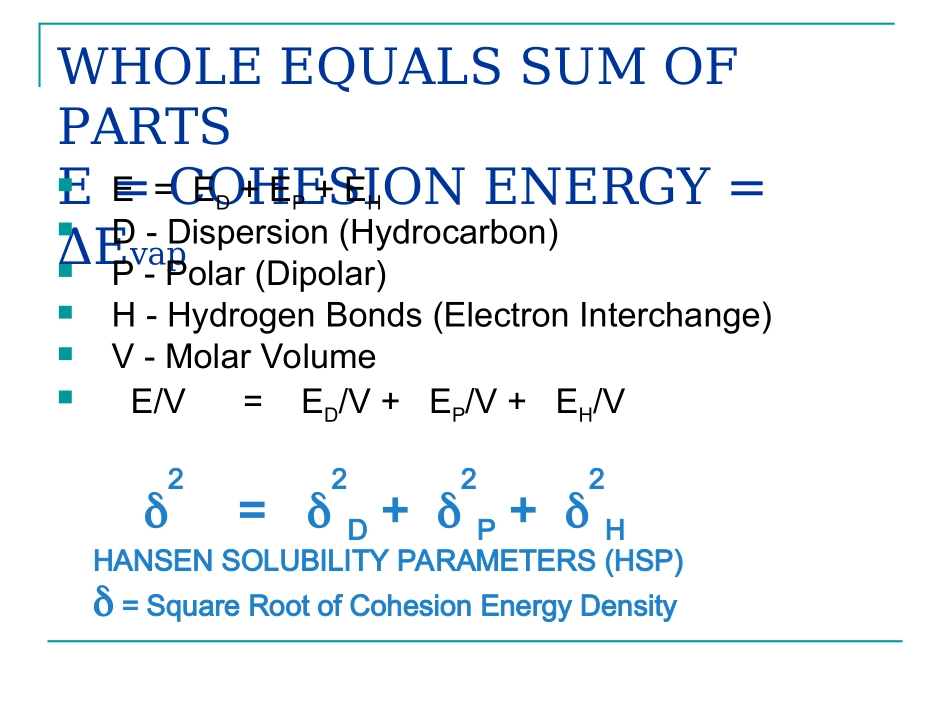
HANSENSOLUBILITYPARAMETERSCHARLESM.HANSENWHYKEEPGOING?”Evenifyou’reontherighttrack,you’llgetrunoverifyoujustsitthere.”-WillRogers-Tomethismeanshelpdevelopthe-HansenSolubilityParametersinPractice-(HSPiP)eBook/softwareWHOLEEQUALSSUMOFPARTSE=COHESIONENERGY=ΔEvapE=ED+EP+EHD-Dispersion(Hydrocarbon)P-Polar(Dipolar)H-HydrogenBonds(ElectronInterchange)V-MolarVolumeE/V=ED/V+EP/V+EH/V2=2D+2P+2HHANSENSOLUBILITYPARAMETERS(HSP)=SquareRootofCohesionEnergyDensityDHOMOMORPHCONCEPT(ED=EFORSIMILARHYDROCARBON)CORRESPONDINGSTATETHEORY(CST)CSTFIGUREFOREDFOREACHOFALIPHATIC,CYCLOALIPHATIC,ORAROMATICSTRUCTUREEDversusVforTr=T298.15./TCRITICALFIGUREFOREDFORALIPHATICHYDROCARBONSPBöttcherEquationcal/cm32222222112108DDPnnVBeerbowerEquationMPa½P=37.4(µ)/V½H1.EH=E-ED-EP2.Panayiotou–statisticalthermodynamicsdirectly3.GroupContributionsH=(EH/V)½4.CHECKwherepossiblethat:2=2D+2P+2HTHERMODYNAMICBASISOFHSPExchangeEnergy(Density)A12=ε11+ε22-2ε12GeometricMeanε12=(ε11ε22)½ScatchardA12=(ε½11-ε½22)2Hildebrand(CohesiveEnergyDensity)ε11=ΔE1/V1;ε22=ΔE1/V1Hildebrand/ScottΔEM=φ1φ2(x1V1+x2V2)(1-2)2Patterson/DelmasΔGnoncomb=φ1φ2VM(1-2)2THERMODYNAMICBASIS(CONT.)HansenHSPRa2=4(D1-D2)2+(P1-P2)2+(H1-H2)2HansenRelativeEnergyDifference(RED)RED=Ra/RoFlory/HansenX/XC=(RED)2Prigogine(WithGeometricMean)ν2=(2Prig/4+9ρ2)wherePrig=(ε2-ε1)/ε1Prigogine/Hansen2Prig=[(i1-i2)/o]2For“i”=P,HPanayiotou-DirectCalculationofHydrogenBondingSTATISTICALTHERMODYNAMICS-PANAYIOTOUEquationofstate:Chemicalpotential:0ln2~~1ln2~~1ln~~00zrqzrlTPHRTRTRTHdpTPrTqzqrqqzlrRTrrdp~~~ln2~~1ln2~ln~1lnHHHHaaadddrRTlnlnPANAYIOTOU2D,2P,and2HVqNrrrdVsrmqNrrrp22VENHHhb2H-COMPARISONHANSENPANAYIOTOUToluene2.002.00Tetralin2.902.90Acetone6.957.00MethylMethacrylate5.405.40Ethanol19.4319.981-Butanol15.8015.80Dimethylsulfoxide10.2010.28Water42.3242.172H–POLYMERCOMPARISONHANSENPANAYIOTOULin.Polyethylene2.802.80Polystyrene2.902.90PVC3.403.42PMMA5.105.10PC6.906.90Nylon6624.0023.90FREEENERGYCHANGE,G,DETERMINESSOLUBILITYORNOTFreeenergyGmustbenegativeforsolutionG=(1/N)øln(ø)+(1-ø)ln(1-ø)+Χø(1-ø)øisthesolventvolumefractionNisthenumberofmonomersinchainΧ=Vm/RT[(D1-D2)2+0.25(P1-P2)2+0.25(H1-H2)2]Χisthechiparameter,VmisthemolarvolumePVERSUSHPLOTHANSENSOLUBILITYPARAMETERDIAGRAMInside:Plasticabsorbs/dissolvesinliquidOutside:Plasticresists/stopsliquid,HydrogenBondingParameter,PolarParameterHansenSolubilityParameterDiagramHpKEYEQUATIONSRa2=4(D1-D2)2+(P1-P2)2+(H1-H2)2Theexperimentallyverified”4”isalsofoundinPrigogine’sCSTtheoryRED=Ra/Ro(Distancetospherecenterdividedbyitsradius)(RED)2=(Ra/Ro)2correspondsto12/cinHuggins/FloryTheorySPHEROIDSOFSOLUBILITYUNLESS”4”ISUSEDEFFECTOFTEMPERATUREHighertemperature–LowervaluesLargereffectforHEPH1E2T2A2T1A1T1T2