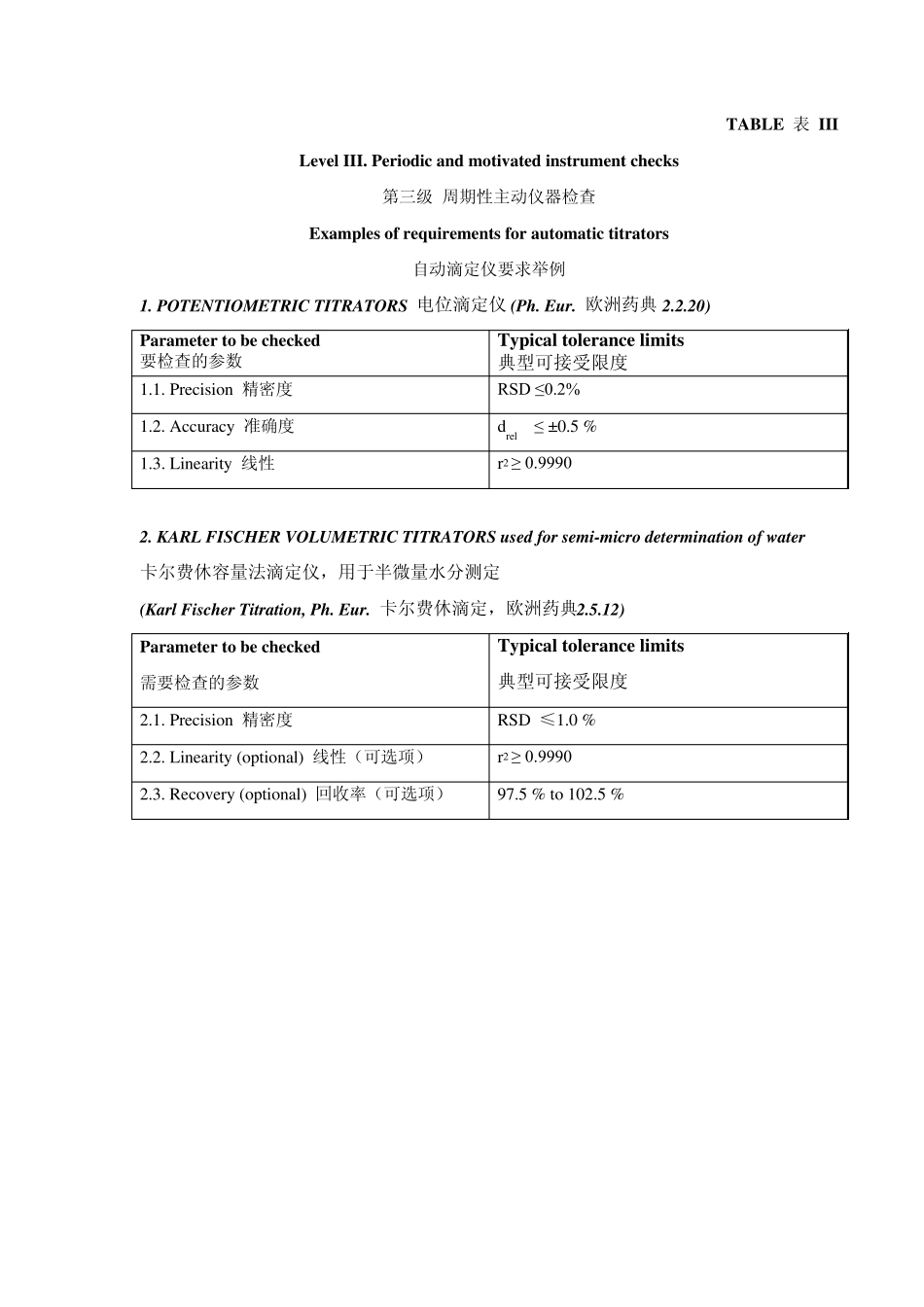
PA/PH/OMCL (07) 108 4R - OMCL Guideline on automatic titrators (Annex 5) OMCL Network of the Council of Europe QUALITY MANAGEMENT DOCUMENT 质量管理文件 PA/PH/OMCL (07) 108 4R QUALIFICATION OF EQUIPMENT 仪器的确认 ANNEX 5: QUALIFICATION OF AUTOMATIC TITRATORS 附件5:自动滴定仪的确认 Full document title and reference 文件全名和参考文献 Qualification of Equipment 仪器的确认 Annex 5: Qualification of automatic titrators 附件5:自动滴定仪的确认 PA/PH/OMCL (07) 108 4R Document type 文件类型 Guideline 指南 Legislative basis 立法基础 The previous version of this document was also accepted by EA as recommendation document to be used in the context of Quality Management System audits of OMCLs Date of first adoption 首次采用日期 June 2008 Date of original entry into force 初版生效日期 August 2008 Date of entry into force of revised document 修订本生效日期 1st May 2012 Previous titles/other References 曾用名/其它参考文献 This document replaces document PA/PH/OMCL (07) 108 3R 本文件替代文件PA/PH/OMCL (07) 108 3R Custodian Organisation 管理机构 The present document was elaborated by the OMCL Network/ EDQM of the Council of Europe Concerned Network 相关网络 GEON ANNEX 5 OF THE OMCL NETWORK GUIDELINE “QUALIFICATION OF EQUIPMENT” OMCL网络指南“仪器确认”附件5 QUALIFICATION OF AUTOMATIC TITRATORS 自动滴定仪的确认 Introdu ction 介绍 The present document is the 5th Annex of the core document “Qualification of Equipment”, and it should be used in combination with it when planning, performing and documenting the qualification process of automatic titrators. 本文件为核心文件“仪器的确认”的第5个附件,在对计划、实施和记录自动滴定仪的确认过程中,应将本文件与核心文件一起使用。 The core document contains the Introduction and general forms for Level I and II of ...