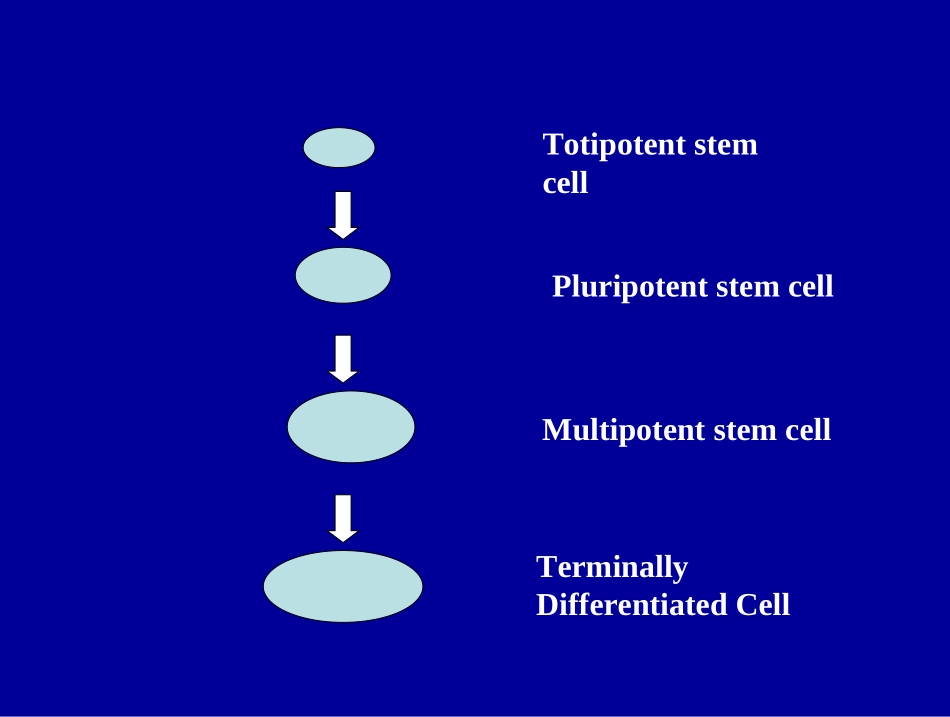
干细胞临床研究现状TotipotentstemcellPluripotentstemcellMultipotentstemcellTerminallyDifferentiatedCell2009年3月奥巴马取消美国对胚胎干细胞研究限制后,推动了干细胞的临床研究。.October11,2010-GeronCorporation(Nasdaq:GERN)todayannouncedtheenrollmentofthefirstpatientinthecompany'sclinicaltrialofhumanembryonicstemcell(hESC)-derivedoligodendrocyteprogenitorcells,GRNOPC1.TheFDAnotificationenablesGerontomoveforwardwiththeworld'sfirstclinicaltrialofahumanembryonicstemcell(hESC)-basedtherapyinman,toachieverestorationofspinalcordfunctionbytheinjectionofhESC-derivedoligodendrocyteprogenitorcellsdirectlyintothelesionsiteofthepatient'sinjuredspinalcord.ReNeuronconfirmedthatthefirstpatienthasbeentreatedinwhatitclaimsistheworld’sfirstfullyregulatedclinicaltrialtoevaluateneuralstemcelltherapyinstrokepatients.2009-11TotipotentstemcellPluripotentstemcellMultipotentstemcellMonopotentstemcell造血干细胞Page1290造血干细胞增殖与分化示意图TBRLorentzEetal(1951)ANNUALNUMBERSOFBLOODANDMARROWTRANSPLANTSWORLDWIDE1970-2000MDM01_21.pptNUMBEROFTRANSPLANTSYEAR19701975198019851990199505,00010,00015,00020,00025,00030,00035,00040,000AutologousAllogeneic2000E.DonnallThomasTheNobelPrizeinPhysiologyorMedicine1990FredHutchinsonCancerResearchCenterSeattle,WA,USA进一步扩充脐血公共库储量5~10万份脐血中,5个位点相合几率(5/6)约80~86%;4个位点相合几率(4/6)约96~97%。NatureMedicine16,232-236(2010)Publishedonline:17January2010|doi:10.1038/nm.2080Notch-mediatedexpansionofhumancordbloodprogenitorcellscapableofrapidmyeloidreconstitutionColleenDelaney1,2,ShellyHeimfeld1,CarolynBrashem-Stein1,HowardVoorhies1,RonaldLManger1&IrwinDBernstein1,2Furthermore,whencordbloodprogenitorsexpandedexvivointhepresenceofNotchligandwereinfusedinaclinicalsettingafteramyeloablativepreparativeregimenforstemcelltransplantation,thetimetoneutrophilrecoverywassubstantiallyshortened.Toourknowledge,thisisthefirstinstanceofrapidengraftmentderivedfromexvivoexpandedstem/progenitorcellsinhumans.进一步发展造血干细胞体外扩增技术FredHutchinsonCancerResearchCenter,Seattle,Washington,USAThemeanfoldexpansionforbothTNCsandCD34+cellsafter16dinculturewithDelta1ext-IgGisdepicted.Theindividualandmediantimes(solidline)toanANCof≥500cellsperμlforpatientsreceivingdouble-unitcordbloodtransplantswithtwounmanipulatedunits(conventional)versusoneexvivo–expandedunitandoneunmanipulatedunit(expanded).*GFP+BMHSC**BM(1.5×107)livermusclelungkidney(1~100)AnalysisofHSC-derivedCellsinsingleHSC-transplantedmice间充质干细胞1970,A.J.Friedenstein,founderofthemesenchymalstemcellconcept间充质干细胞(MSC)MSC主要分布于骨髓、脐血、脂肪、牙髓、肌肉以及众多的结缔组织,可以分化为脂肪细胞、成骨细胞、软骨细胞等。MSC起到重要的免疫调节作用,是骨髓微环境的重要组成等。MSC是一个不均一的细胞群体,由于采用不同的细胞分离和扩增技术、制定不同的质量标准,可能得到不同的结果。2006年国际细胞治疗协会间充质和组织干细胞委员会提出了制定MSC的标准:Plasticadherent●间充质干细胞增殖分化示意图CD90,CD105CD73,(+)对于异基因间充质干细胞治疗,具有以下特点:1.脐带是人体废弃物,采集它的细胞不涉及人权、隐私等伦理问题。当然,患者对上述细胞治疗有知情权,收集脐带须得到供者的书面同意。2.以骨髓和脐带来源的间充质干细胞为例,截止至2011年3月,美国FDA已批准应用MSC进行临床研究的试验方案共154项,其中已完成I期临床安全性试验的方案共有109项,有2项已完成III临床试验,一项是MSC治疗急性移植物抗宿主病(GVHD),一项是MSC治疗克隆氏病。间充质...