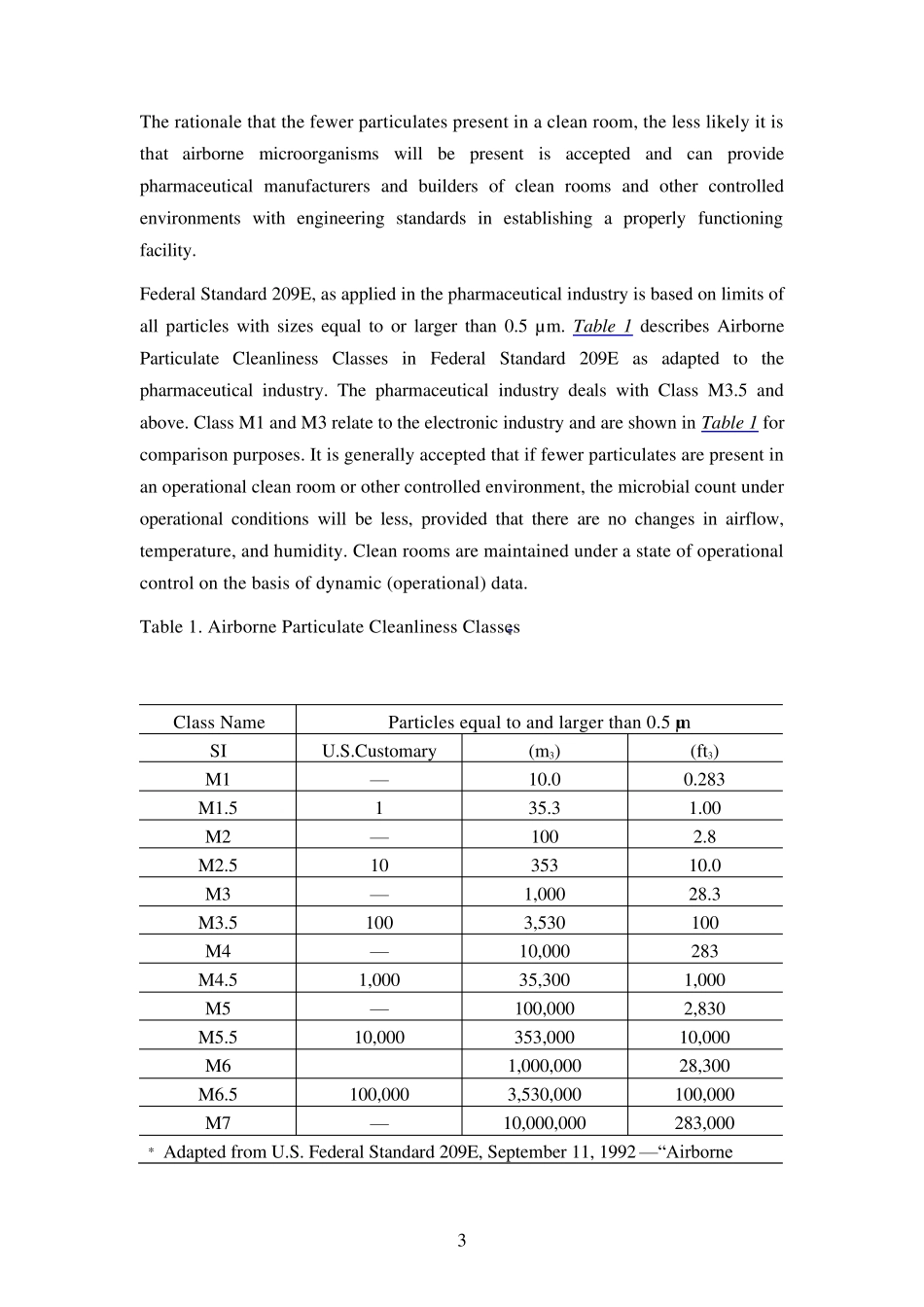
1 <1116> MICROBIOLOGICAL EVALUATION OF CLEAN ROOMS AND OTHER CONTROLLED ENVIRONMENTS (美国药典28 版) 洁净室和其他受控环境的微生物检测 The purpose of this informational chapter is to review the various issues that relate to aseptic processing of bulk drug substances, dosage forms, and in certain cases, medical devices; and to the establishment, maintenance, and control of the microbiological quality of controlled environments. 本章节旨在回顾各种关于原料药物、制剂以及某些医疗器械的无菌处理,和对受控环境微生物质量的确立、维持和控制。 This chapter includes discussions on (1) the classification of a clean room based on particulate count limits; (2) microbiological evaluation programs for controlled environments; (3) training of personnel; (4) critical factors in design and implementation of a microbiological evaluation program; (5) development of a sampling plan; (6) establishment of microbiological Alert and Action levels; (7) methodologies and instrumentation used for microbiological sampling; (8) media and diluents used; (9) identification of microbial isolates; (10) operational evaluation via media fills; and (11) a glossary of terms. Excluded from this chapter is a discussion of controlled environments for use by licensed pharmacies in the preparation of sterile products for home use, which is covered under Pharmaceu tical Compou nding—Sterile Preparations á797ñ. 本章节包 括 根 据 微粒 限 度 制定 的洁净室级 别 的讨 论 (1),受控环境的微生物分析 程 序 (2),人 员 培 训 (3),在设 计 和执 行 微生物分 析 程 序 时 的临 界 因 素(4),抽 样 检验 方 法 的改 进 (5),微生物预 警 和应 对级 别 的确立(6),微生物取 样 的方 法 学 和实 验 仪 器(7),使 用 的中 间 体 和稀 释 药液 (8), There are alternative methods to assess ...