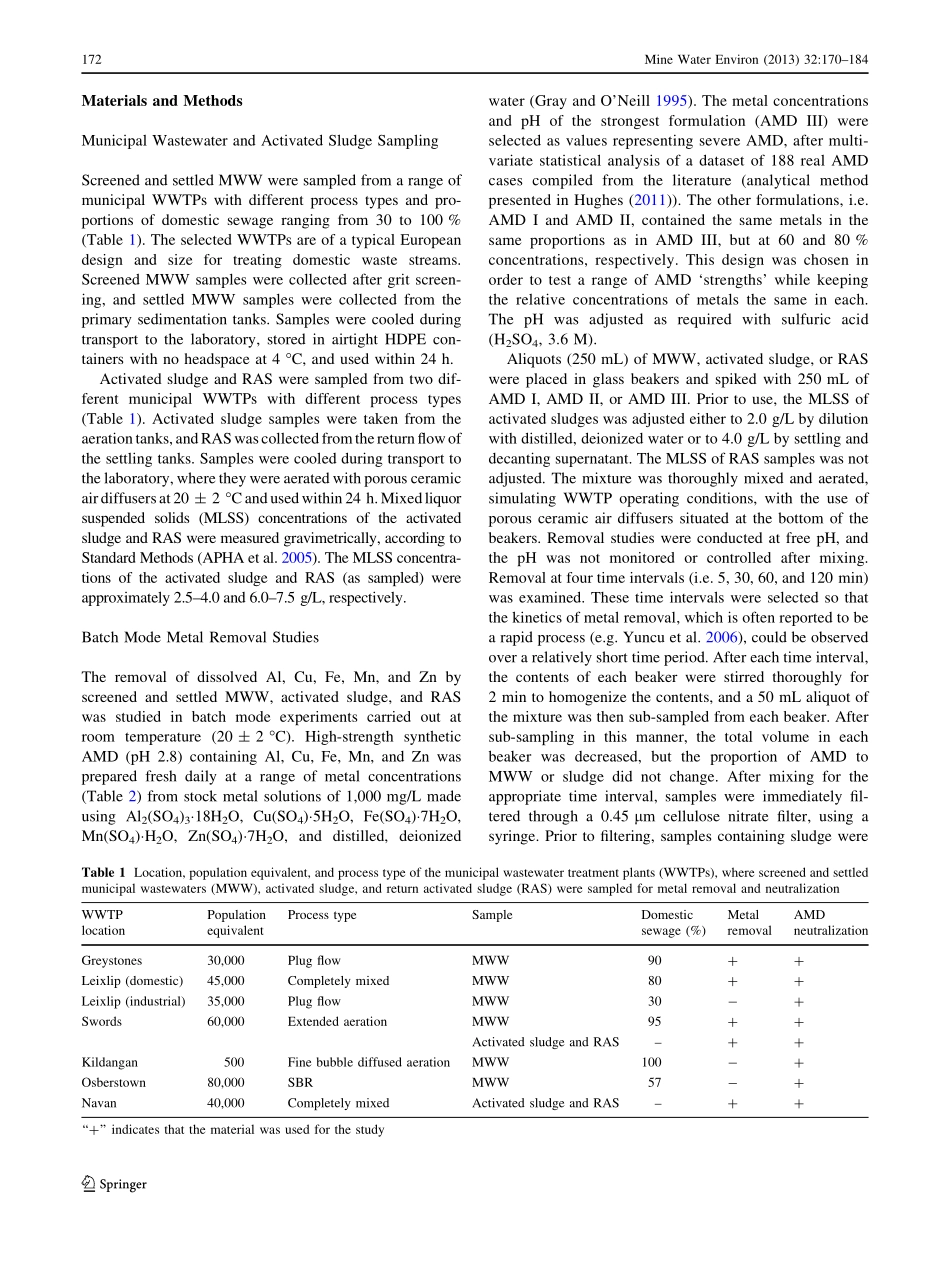
TECHNICALARTICLERemovalofMetalsandAcidityfromAcidMineDrainageUsingMunicipalWastewaterandActivatedSludgeTheresaA.Hughes•N.F.GrayReceived:24August2012/Accepted:20February2013/Publishedonline:5March2013�Springer-VerlagBerlinHeidelberg2013AbstractCo-treatmentofacidminedrainage(AMD)andmunicipalwastewater(MWW)usingtheactivatedsludgeprocessisaninnovativeapproachtoAMDremediationthatutilizesthealkalinityofMWWandtheadsorptiveprop-ertiesofthewastewaterparticulatesandactivatedsludgebiomasstobufferacidityandremovemetals.ThecapacityofthesematerialstotreatAMDwasinvestigatedinbatchmodemetalremovaltestsusinghigh-strengthsyntheticAMD(pH2.8,Al120–200mg/L,Cu18–30mg/L,Fe324–540mg/L,Mn18–30mg/L,andZn36–60mg/L).UsingmaterialfromarangeofMWWtreatmentplants,theperformanceofscreenedandsettledMWW,activatedsludgeswithmixedliquorsuspendedsolids(MLSS)con-centrationsof2.0and4.0g/L,andreturnactivatedsludgeswith6.0and7.4g/LMLSSwerecompared.SimilartrendswereobservedfortheMWWandactivatedsludges,withremovalefficiencygenerallydecreasingintheorderAl=Cu[Mn[Zn[Fe.TrendsinFeremovalusingsettledMWWandactivatedsludgeswerehighlyvariable,withremoval\30%.Usingactivatedsludges,averageremovalefficienciesforAl,Cu,Mn,andZnwere10–65%,20–60%,10–25%,and0–20%,respectively.Sludgesolidsconcentrationwasanimportantcontrollingfactorinmetalremoval,withremovalofAl,Cu,Mn,andZnincreasingsignificantlywithsolidsconcentration.Munici-palwastewatershadgreaterneutralizationcapacitiesthanactivatedsludgesathighAMDloadingratios.MixingAMDwithscreenedMWWgavethehighestremovalefficiencyforallmetals,achievingaverageremovalof90–100%forAl,Cu,andFe,65–100%forZn,and60–75%forMn.Theseempiricalfindingsareusefulfordevelopingprocessdesignparametersinco-treatmentsystems.UtilizingMWWandactivatedsludgetoremediateAMDcanpotentiallyreducematerialsandenergyrequirementsandassociatedcosts.KeywordsAdsorption�Co-treatment�Neutralization�Treatment�WastewatertreatmentplantIntroductionAcidminedrainage(AMD)generatedduringtheoxidationofmineralsurfacesexposedduringmineralextractionleadstothereleaseofdissolvedmetals,sulfate,andhydrogenions(StummandMorgan1981)intooverlyingorinfiltratingwatersandcanseverelydegradewaterqualityinsurfacewatersandunderlyingaquifersinthevicinityofthemine(Mlayahetal.2009;WolkersdorferandBowell2004).ThekeytreatmentprocessesrequiredtoremediateAMDaremetalremovalandacidneutralization,bothofwhicharecomplexprocessesthatdependonmanyfactors,includingconcentrationandsolubilityofmetalions,andpH(Evangelou1998).Removalofacidity,metals,andsulfateinactiveandpassiveAMDtreatmentisaccom-plishedbydosingwithalkalinesubstances(e.g.CaO)orgeneratingalkalinityeitherabioticallyviapassivedisso-lutionoflimestoneorbioticallyviabacterialsulfatereduction(Hedinetal.1994;Skousenetal.1998;Watzlafetal.2004).TheseprocessesincreasepHandaidintheremovalofdissolvedmetalsbyprecipitationand/oradsorption.ThepHhasamajorinfluenceonprecipitationandmetaladsorption;competitionoccursbetweenH?andmetalionsforbindingsites,andmetalremovalbyadsorptionisoftenreportedtoincreasewithpHT.A.Hughes(&)�N.F.GrayWaterTechnologyResearchGroup,CentrefortheEnvironment,SchoolofNaturalSciences,TrinityCollege,Dublin2,Irelande-mail:hughesta@tcd.ie123MineWaterEnviron(2013)32:170–184DOI10.1007/s10230-013-0218-8(HawariandMulligan2006;Motsietal.2009;Rozadaetal.2008;Zhang2011).Municipalwastewater(MWW)i...