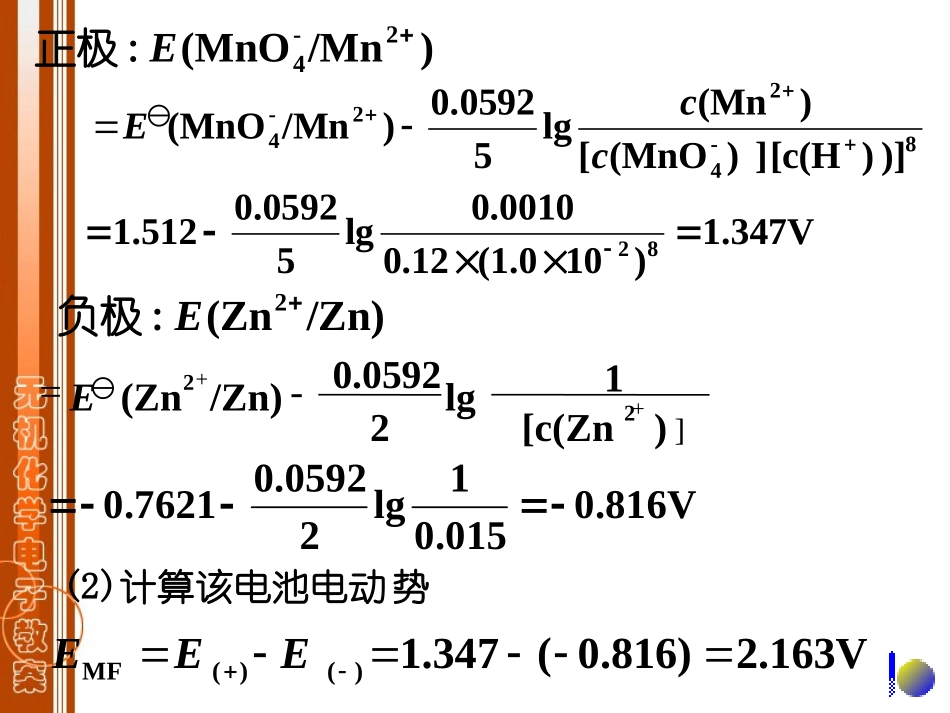
)()(cc,氧化型还原型)(c,还原型)(c,氧化型2.影响电极电势的因素e还原型氧化型电极反应:Zlg)()(氧化型还原型cbcaZ0.0592EE或E则:①氧化型或还原型的浓度或分压例有一原电池:298KT,L0.015mol)(Zn,L0.0010mol)(Mn,L0.12mol)(MnO2.00,pH-12-12-1-4ccc若(1)两电极电极电势:计算:电极反应为,负极为,正极为:解/ZnZn/MnMnO22-4时,:查表得298K)/Mn(MnO2-4E1.512V,0.7621V/Zn)(Zn2E1Lmol101.0)(H2.00,pH2cZn(S)2e(aq)ZnO(l)4H(aq)Mn5e(aq)8H(aq)MnO2224pt(aq)(aq),Mn(aq)ZnZn22MnO4)/Mn(MnO2-4E:正极8-422-4)])[c(H])(MnO[)(Mnlg50.0592)/Mn(MnOccE1.347V)10(1.00.120.0010lg50.05921.51282/Zn)(Zn2E:负极0.0592lg2/Zn)(ZnE2])[c(Zn21++-=0.816V0.0151lg20.05920.7621势(2)计算该电池电动MF()()1.347(0.816)2.163VEEE)/ClClO(3E?)/ClClO(时L10.0mol)H(31Ec,L1.0mol)Cl()ClO(13cc,求:当②介质的酸碱性V45.1)/ClClO(3E已知例:3)}Cl({)}H()}{ClO({lg60.0592V)/ClClO(63cccE)l(O3H)aq(Cl6e)aq(6H)aq(ClO解:23V51.10.10lg6V0592.061.45VAg1L1.0mol)Cl(cAg③沉淀的生成对电极电势的影响,)108.1)AgCl((?Ag)/(AgClsAgClNaClAgAgV799.0Ag)/(Ag10spKEE?Ag)/(AgLmol0.1)Cl(1Ec并求时,平衡后会产生加入电池中组成的半和,若在已知例:0.222V108.1lgV0592.00.799V10)Ag/Ag(E)aq(Cl)aq(Ag(s)AgCl解:Ag(s)e)aq(Ag(AgCl))}Cl()}{Ag({spKcc(AgCl))Ag(,Lmol0.1)Cl(sp1时若Kcc)}Ag({lgV0592.0)Ag/Ag(cEAgCl)(lgV0592.0)Ag/Ag(spKEV222.0)/AgAg(E)aq(ClAg(s)eAgCl(s)1,Lmol0.1)Cl(时当c)/AgAgCl(E=AgCl)(lgV0592.0)Ag/Ag(spKENaOH,达到平衡时保持的半电池中加入?)FeFe(,Lmol0.1)OH(231求此时Ec,108.2)(OH)Fe(393spK例:V769.0)FeFe(23,已知EFeFe1086.4)(OH)Fe(23172sp组成和,在K?)(OH)Fe/Fe(OH)(23E解:在Fe3+、Fe2+的溶液中加入NaOH后,时L1.0mol)OH(1当c)aq(3OH)aq(Fe(s)(OH)Fe33(s)(OH)Fe2)aq(2OH)aq(Fe23-33SP)])/(OH[])/(Fe[1)(Fe(OH)1ccccKK12-22SP2)])/(OH[])/(Fe[1)(Fe(OH)1ccccKK)(Fe(OH))(Fe3SP3Kcc)(Fe(OH))(Fe2SP2KccV55.01086.4lg0592.0V769.01739108.2)aq(Fee)aq(Fe23)Fe(OH)(lg0592.0)Fe/Fe(2sp23KE)Fe(OH)(3spK)Fe/Fe(23EccccE)/(Fe)/(Fe0.0592lg)/Fe(Fe32230.55V.055V)/FeFe(23E,Lmol0.1)OH(1时当c)(OH)/Fe(OH)(Fe23即ElgV0592.0)/FeFe(23E)(OH)(Fe3spK)(OH)(Fe2spK)(OH)/Fe(OH)(Fe23E)aq(OH)s((OH)Fee)s((OH)Fe23小结:氧化型形成沉淀,E↓;还原型形成沉淀,E↑。)Ag/Ag(/Ag)S(Ag2例:EE)Cu/Cu(/CuI)(Cu22EE氧化型和还原型都形成沉淀,看二者的相对大小。若(氧化型)<(还原型),则E↓;反之,则E↑。KspKspKsp第五次作业P1167-15——(2),(3)7-16