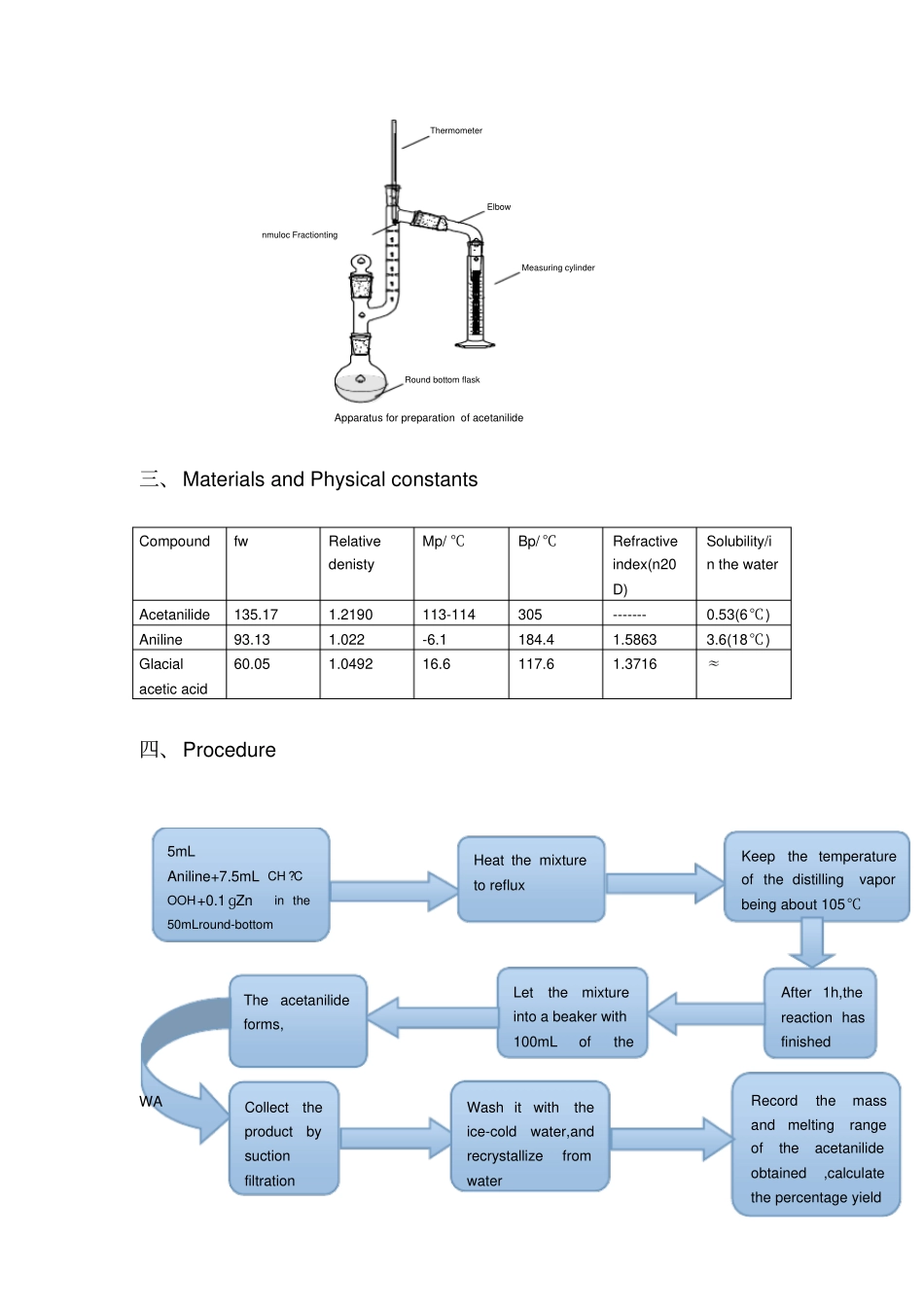
Amidation of Carboxylic Acids:preparation of Acetanilide Chen Xiaoling Class2 2012364217 一、Purpeses and Requirements ⑴Learn the principles and operation of aniline aceltylation.⑵Consolidate the recrystallization method and the principle of purflication of organic compounds. 二、The experimental princinple and devices. Principle Acetanilide can be prepared from aniline in several ways, using acetyl chloride,acetic anhydride or glacial acetic acid as starting materials. Acetyl chloride reacts very vigorously. Acetican hydride is preferred for a laboratory synthesis because its rate of hydrolysis is low enough to allow the acetylation of amine to be carried out in aqueous solution. It gives a product of high purity and in good yield. The procedure with glacial is of commercial interest since it is economical, but it requires long heating. In the present experiment, we can use either of the following methods to prepare the acetanilide. The main reaction: NH2CH3COOHNHCOCH3H2ODevices Round bottom flasknmuloc FractiontingElbowThermometerMeasuring cylinderApparatus for preparation of acetanilide三、Materials and Physical constants Compound fw Relative denisty Mp/ ℃Bp/ ℃Refractive index(n20D) Solubility/in the water Acetanilide 135.17 1.2190 113-114 305 ------- 0.53(6℃) Aniline 93.13 1.022 -6.1 184.4 1.5863 3.6(18℃) Glacial acetic acid 60.05 1.0492 16.6 117.6 1.3716 ≈四、Procedure WA 5mL Aniline+7.5mL CH ?COOH+0.1 ɡZn in the 50mLround-bottom Heat the mixture to reflux Keep the temperature of the distilling vapor being about 105℃After 1h,the reaction has finished Let the mixture into a beaker with 100mL of the The acetanilide forms, Collect the product by suction filtration Wash it with the ice-cold water,and recrystallize fro...