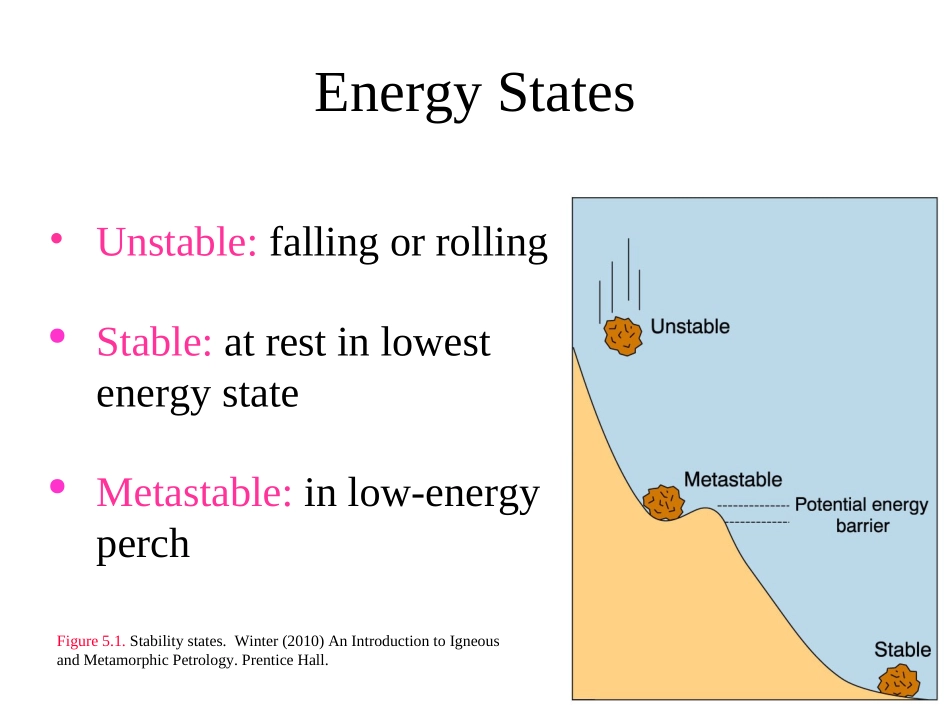
ThermodynamicsBeginwithabriefreviewofChapter5NaturalsystemstendtowardstatesofminimumenergyEnergyStates•Unstable:fallingorrollingStable:atrestinlowestenergystateMetastable:inlow-energyperchFigure5.1.Stabilitystates.Winter(2010)AnIntroductiontoIgneousandMetamorphicPetrology.PrenticeHall.GibbsFreeEnergyGibbsfreeenergyisameasureofchemicalenergyGibbsfreeenergyforaphase:G=H-TSWhere:G=GibbsFreeEnergyH=Enthalpy(heatcontent)T=TemperatureinKelvinsS=Entropy(canthinkofasrandomness)ThermodynamicsGforareactionofthetype:2A+3B=C+4DG=(nG)products-(nG)reactants=GC+4GD-2GA-3GBThesideofthereactionwithlowerGwillbemorestableThermodynamicsForothertemperaturesandpressureswecanusetheequation:dG=VdP-SdT(ignoringXfornow)whereV=volumeandS=entropy(bothmolar)WecanusethisequationtocalculateGforanyphaseatanyTandPbyintegratingzzGGVdPSdTTPTPTTPP21112122IfVandSareconstants,ourequationreducesto:GT2P2-GT1P1=V(P2-P1)-S(T2-T1)Nowconsiderareaction,wecanthenusetheequation:dG=VdP-SdT(againignoringX)Gforanyreaction=0atequilibriumWorkedProblem#2used:dG=VdP-SdTandG,S,Vvaluesforalbite,jadeiteandquartztocalculatetheconditionsforwhichGofthereaction:Ab+Jd=Qisequalto0fromGvaluesforeachphaseat298Kand0.1MPacalculateG298,0.1forthereaction,dothesameforVandSGatequilibrium=0,sowecancalculateanisobaricchangeinTthatwouldberequiredtobringG298,0.1to00-G298,0.1=-S(Teq-298)(atconstantP)Similarlywecouldcalculateanisothermalchange0-G298,0.1=-V(Peq-0.1)(atconstantT)MineralS(J)G(J)V(cm3/mol)LowAlbite207.25-3,710,085100.07Jadeite133.53-2,844,15760.04Quartz41.36-856,64822.688FromHelgesonetal.(1978).Table27-1.ThermodynamicDataat298Kand0.1MPafromtheSUPCRTDatabaseMethod:NaAlSi3O8=NaAlSi2O6+SiO2P-TphasediagramoftheequilibriumcurveHowdoyouknowwhichsidehaswhichphases?Figure27.1.Temperature-pressurephasediagramforthereaction:Albite=Jadeite+QuartzcalculatedusingtheprogramTWQofBerman(1988,1990,1991).Winter(2010)AnIntroductiontoIgneousandMetamorphicPetrology.PrenticeHall.pickanytwopointsontheequilibriumcurvedG=0=VdP-SdTThusdPdTSVFigure27.1.Temperature-pressurephasediagramforthereaction:Albite=Jadeite+QuartzcalculatedusingtheprogramTWQofBerman(1988,1990,1991).Winter(2010)AnIntroductiontoIgneousandMetamorphicPetrology.PrenticeHall.ReturntodG=VdP-SdT,foranisothermalprocess:GGVdPPPPP2112zGasPhasesGasPhasesForsolidsitwasfinetoignoreVasf(P)ForgasesthisassumptionisshittyYoucanimaginehowagascompressesasPincreasesHowcanwedefinetherelationshipbetweenVandPforagas?GasPressure-VolumeRelationshipsIdealGas–AsPincreasesVdecreases–PV=nRTIdealGasLaw•P=pressure•V=volume•T=temperature•n=#ofmolesofgas•R=gasconstant=8.3144Jmol-1K-1PxVisaconstantatconstantTFigure5.5.Piston-and-cylinderapparatustocompressagas.Winter(2010)AnIntroductiontoIgneousandMetamorphicPetrology.PrenticeHall.GasPressure-VolumeRelationshipsSincewecansubstituteRT/PforV(forasinglemoleofgas),thus:and,sinceRandTarecertainlyindependentofP:GGVdPPPPP2112zGGRTPdPPPPP2112zzGGRTPdPPPPP21121GasPressure-VolumeRelationshipsAndsinceGP2-GP1=RTlnP2-lnP1=RTln(P2/P1)ThusthefreeenergyofagasphaseataspecificPandT,whenreferencedtoastandardatateof0.1MPabecomes:GP,T-GT=RTln(P/Po)GofagasatsomePandT=Ginthereferencestate(sameTand0.1MPa)+apressureterm1xdxxzlnoGasPressure-VolumeRelationshipsTheformofthisequationisveryusefulGP,T-G...