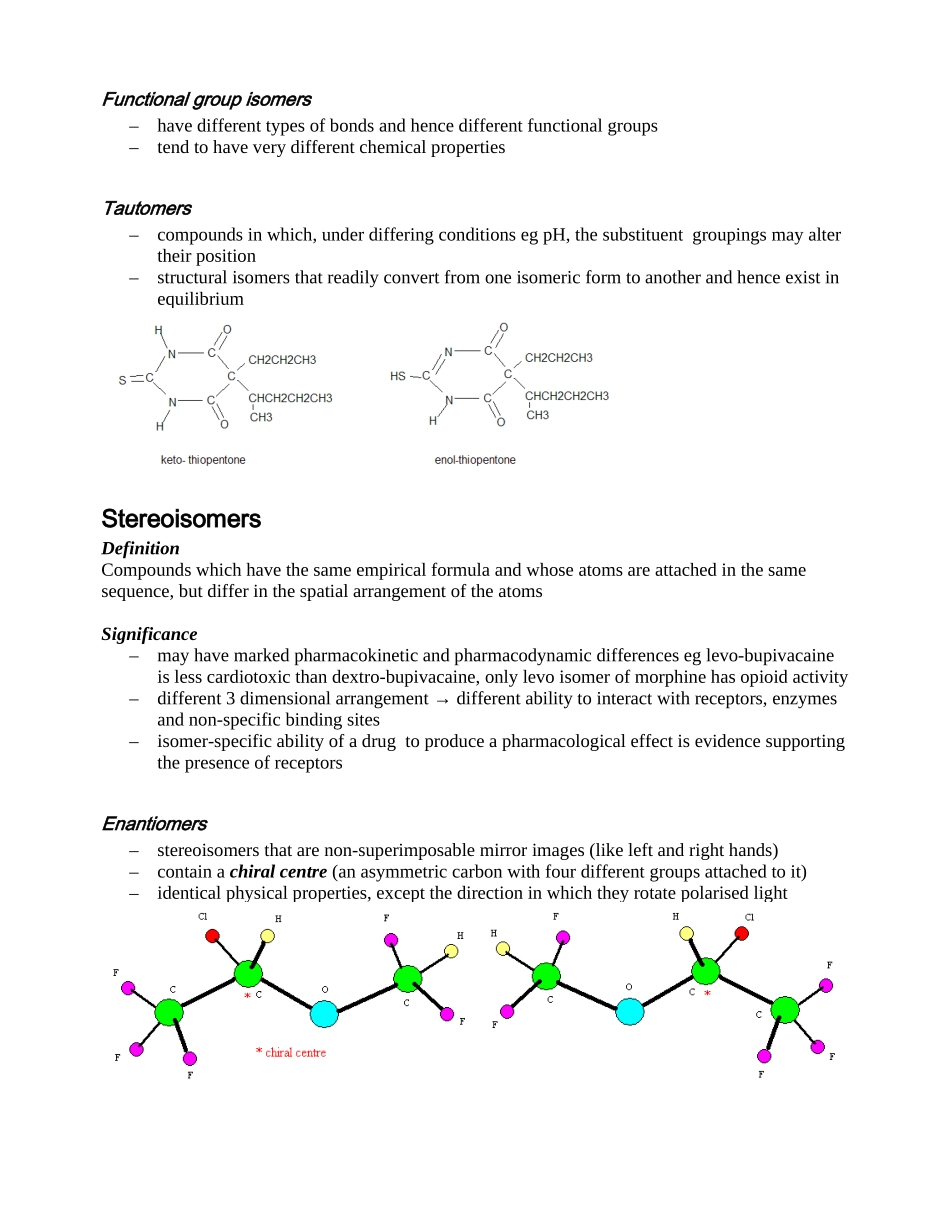
ISOMERSDefinitionIsomersarecompoundsthathavethesamemolecular(empirical)formula,butdifferentstructures,anddemonstratephysico-chemicalandpharmacologicaldifferencesClassificationStructuralIsomersDefinitionCompoundswiththesameempiricalformula,butwhoseatomsareconnectedinadifferentsequenceChainisomers–havedifferentbranchingpatternsofcarbonchainsPositionisomers–thefunctionalgroupislocatedondifferentcarbonsinthechain–tendtohavesimilarchemicalpropertiesFunctionalgroupisomers–havedifferenttypesofbondsandhencedifferentfunctionalgroups–tendtohaveverydifferentchemicalpropertiesTautomers–compoundsinwhich,underdifferingconditionsegpH,thesubstituentgroupingsmayaltertheirposition–structuralisomersthatreadilyconvertfromoneisomericformtoanotherandhenceexistinequilibriumStereoisomersDefinitionCompoundswhichhavethesameempiricalformulaandwhoseatomsareattachedinthesamesequence,butdifferinthespatialarrangementoftheatomsSignificance–mayhavemarkedpharmacokineticandpharmacodynamicdifferenceseglevo-bupivacaineislesscardiotoxicthandextro-bupivacaine,onlylevoisomerofmorphinehasopioidactivity–different3dimensionalarrangement→differentabilitytointeractwithreceptors,enzymesandnon-specificbindingsites–isomer-specificabilityofadrugtoproduceapharmacologicaleffectisevidencesupportingthepresenceofreceptorsEnantiomers–stereoisomersthatarenon-superimposablemirrorimages(likeleftandrighthands)–containachiralcentre(anasymmetriccarbonwithfourdifferentgroupsattachedtoit)–identicalphysicalproperties,exceptthedirectioninwhichtheyrotatepolarisedlightClassificationSystemsa)Rotationofpolarisedlighttothe:left:levorotatoryl-(-)right:dextrototatoryd-(+)aracemicmixturecontainsequalamountsoflevoanddextroisomersandthereforehasnooverallrotatingeffectonpolarisedlightb)CahnIngoldPrelogconventionLigandsaroundthechiralcarbonareassignedaprioritybasedontheiratomicnumber(higheratomicnumber=higherpriority)Rectus(R-)prioritiesincreaseinclockwisedirectionSinister(S-)prioritiesincreaseinanti-clockwisedirectionNotimportanttoknowtheexactpriorityrules.Notethatthissystemhasnothingtodowithrotationofpolarisedlightandthereforeclassificationinonesystemdoesnotalwayscorrespondtothesameclassificationintheother.“Coincidentally”,thisseeminglyarbitrarynomenclature(R,S)aretheinitialsofMrR.S.Cahn.c)SimplesugarsandaminoacidscanbeclassifiedasD-orL-accordingtotheclockwiseoranticlockwisespatialarrangementofCOOH,NH3,Handhydrocarbonchainaroundthechiralcarbon.Thisisanoldclassificationsystem,don’tbotherlearningit,justbeawareofit.(D-andL-arenottobeconfusedwithd-andl-!!)Diastereoisomers–Stereoisomerswithdifferentorientationofsubstituentgroupsoneithersideofarigidbond(egdoublebondorringstructure)–Alternatively,canbethoughtofasisomerswithtwochiralcarbonsClassificationsystemsa)cis-andtrans-cis-functionalgroupsareonthesamesideofthedoublebondtrans-functionalgroupsareonoppositesidesofthedoublebondegmivacuriumispresentedasamixtureofisomers:trans-trans60%cis-trans30%cis-cis10%b)othersystemsincludesyn-/anti-andZ-/E-(forgetaboutthiscrap)Moredetailedinfoonisomerscanbefoundathttp://www.wikipedia.orgClassificationofIsomersIsomers:Compoundsthathaveidenticalchemicalandmolecularformulasbutdifferinthenatureofsequenceofbondingoftheiratomsorintheirarrangementofatomsinspace.Accordingtotheirtopologytheyareclassifiedaseitherstructuralorstereoisomers.Isomersarebroadl...