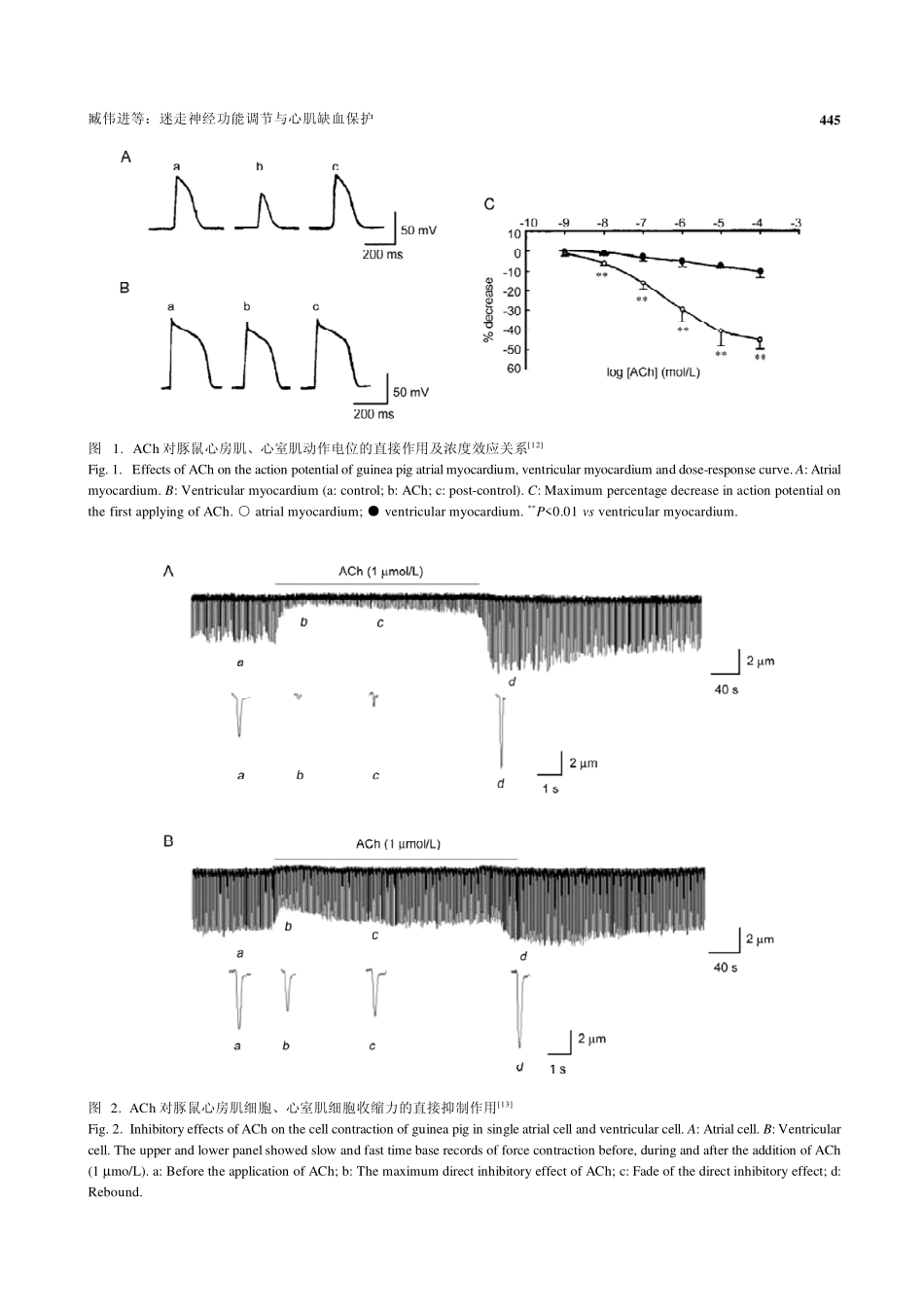
生理学报ActaPhysiologicaSinica,August25,2008,60(4):443-452http://www.actaps.com.cn443国家科学基金成果综述Received2008-03-18Accepted2008-04-25ThisworkwassupportedbygrantsfromtheNationalBasicResearchDevelopmentProgramofChina(973Program)(No.2007CB512005),theNationalNaturalScienceFoundationofChina(No.30770785,30470633,30270554,39870334),theCultivationFundoftheKeyScientificandTechnicalInnovationProjectofMinistryofEducation,China(No.705045)andtheSpecializedResearchFundfortheDoctoralProgramofHigherEducation,China(No.20050698012).*Correspondingauthor.Tel:+86-29-82655003,+86-29-82655150;Fax:+86-29-82655003;Email:zwj@mail.xjtu.edu.cn迷走神经功能调节与心肌缺血保护臧伟进1,2,*,孙蕾1,于晓江1,吕军1,陈莉娜1,刘兵行1西安交通大学1医学院药理学系心血管生理药理研究室;2环境与疾病相关基因教育部重点实验室,西安710061摘要:心血管系统的生理活动受自主神经系统(autonomicnervoussystem,ANS)调节。已有研究表明,自主神经功能紊乱,尤其是迷走神经功能低下,与心血管疾病(cardiovasculardisease,CVD)的发生、发展及预后密切相关。本文结合国内外研究现状,就本研究室在迷走神经对心脏不同部位的调控及其对心肌的保护作用机制方面的研究成果进行阐述。通过收缩功能检测及标准玻璃微电极细胞内记录技术,发现迷走神经递质——乙酰胆碱对哺乳动物心室肌有直接作用,可抑制细胞收缩力及动作电位时程;通过组织化学染色及分子生物学方法进一步证明心室有毒蕈碱受体分布;通过膜片钳技术显示在部分动物心室肌上存在乙酰胆碱激活的内向整流钾通道(acetylcholine-activatedpotassiumchannel,KACh),并且其电流(IK·ACh)和心房肌一样具有衰减现象。前期研究证明心房肌IK·ACh的衰减与毒蕈碱受体、G蛋白或钾通道磷酸化有关;而心室肌的IK·ACh还有待于进一步研究。我们建立了相关动物模型,结合心率变异性分析等自主神经评价方法,探讨ANS在健康和疾病状态下的变化情况,证明了迷走神经对心脏调节的增龄性改变及代偿效应。通过提高迷走张力(乙酰胆碱缺血预/后适应、有氧运动、β受体阻断剂),研究改善自主神经平衡对缺血心肌的保护作用以及胆碱能抗炎通路防御缺血/再灌注诱导的炎症损伤机制。综合评价心脏自主神经调节,改善交感和迷走张力平衡,将为CVD防治的基础研究提供重要的理论依据。关键词:迷走神经;缺血性心脏病;心肌保护;炎症反应中图分类号:R331VagalcontrolofcardiacfunctionsandvagalprotectionofischemicmyocardiumZANGWei-Jin1,2,*,SUNLei1,YUXiao-Jiang1,LVJun1,CHENLi-Na1,LIUBing-Hang11DivisionofCardiovascularPhysiologyandPharmacology,DepartmentofPharmacology,SchoolofMedicine;2KeyLaboratoryofEnvironmentandGenesRelatedtoDiseases,MinistryofEducation,Xi’anJiaotongUniversity,Xi’an710061,ChinaAbstract:Thephysiologicalactivitiesofthecardiovascularsystemareunderthecontrolofautonomicnervoussystem(ANS).Recentresearcheshavefoundthatautonomicdysfunction,especiallythewithdrawalofvagalactivity,wascloselyrelatedtotheetiology,courseandprognosisofcardiovasculardisease(CVD).Basedonthecurrentstatusandourachievementsinthisarea,wediscussvagalregulationofdifferentpartsoftheheartandthemechanismofvagalprotectionofmyocardium.Usingaforcetransducerandstandardmicroelectrodesrecordingtechnology,wefoundthatthevagusnervetransmitter–acetylcholine(ACh)haddirecteffectsonventricularmyocytesinmammals:Itinhibitedthecontractilityandshortenedtheactionpotentialdurationofcardiacmyocytes.Weprovedtheexistenceofmuscarinicreceptorsandvagalnervesinnervationinventriclewithhistochemicalstainingandmolecularbiologicalmethods.Furthermore,ACh-activatedpotassiumchannel(KACh)wasfoundintheventriclesofsomeanimalsbypatch-clamp.Fadeofthecurrent(IK·ACh)toAChin...