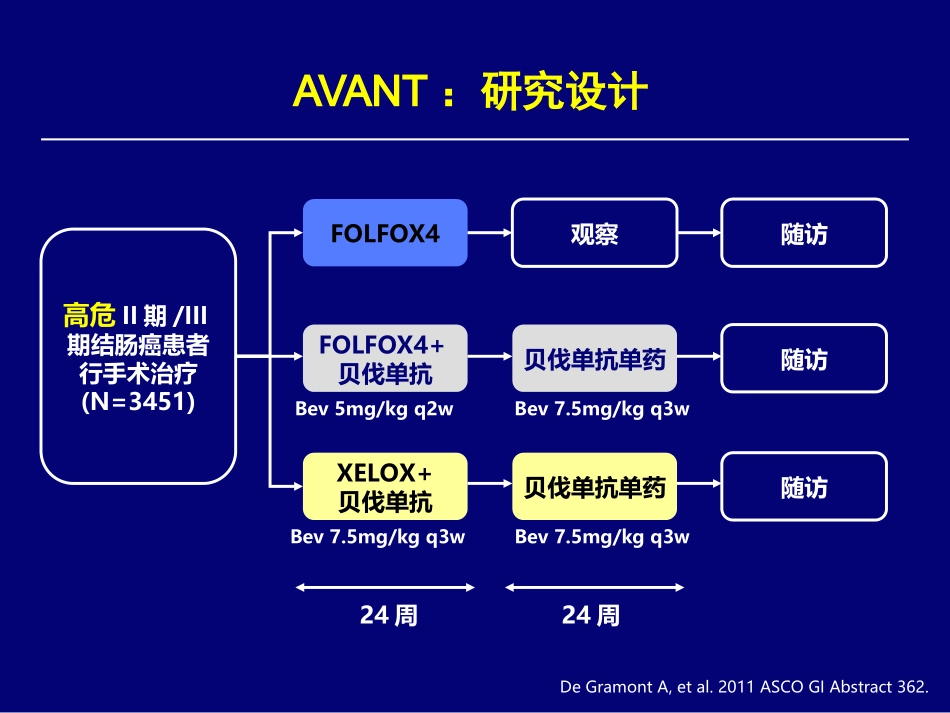
张俊上海交通大学医学院附属瑞金医院外科、肿瘤放化疗科上海消化外科研究所ASCO-GI2011(18-20Jan2011,SF)ASCO胃肠道会议进展Highlightsof2011ASCOGI结直肠癌辅助化疗–Abstract#362:AVANT(XELOX+BVZVSFOLFOX+BVZVSFOLFOXalone)–Abstract#363:NO147(FOLFIRIR±西妥昔单抗)一线化疗–Abstract#365:NORDICVII(FLOX+西妥昔单抗一线治疗mCRC)–打打停停:mCRC标准治疗后以贝伐珠单抗+卡培他滨维持治疗安全有效AVANT:研究设计高危II期/III期结肠癌患者行手术治疗(N=3451)FOLFOX4观察随访FOLFOX4+贝伐单抗贝伐单抗单药随访XELOX+贝伐单抗贝伐单抗单药随访Bev5mg/kgq2wBev7.5mg/kgq3wBev7.5mg/kgq3wBev7.5mg/kgq3w24周24周DeGramontA,etal.2011ASCOGIAbstract362.研究执行情况和终点指标330centers,34countries,8regions(stratified)3451patientsrandomizedbetween20December2004and08June2007-2867patientswithStageIIIdiseasePrimaryendpoints(StageIIIpatientsonly):DFS:FOLFOX4+bevacizumabvs.FOLFOX4DFS:XELOX+bevacizumabvs.FOLFOX4Secondaryendpoints:OSSafetyNon-inferiorityofDFSandOSforFOLFOX4+bevacizumabvs.XELOX+bevacizumab(ifco-primaryendpointsmet)DFS(ITTStageIII)Datacut-offdate:30June2010(3-yearminimumfollow-up)FOLFOX(N=955)FOLFOX4+Bev(N=960)XELOX+Bev(N=952)HR(95%CI)1.17(0.98,1.39)1.07(0.90,1.28)955960952890921900823868865779791784740728722708695688451436415FOLFOX4FOLFOX4+BevXELOX+BevNumberatrisk609586580282280268FOLFOX4FOLFOX4+BevXELOX+BevEvent-freerate0.00.10.20.30.40.50.60.70.80.91.06183036424801224Time(months)54606672121123110010323328000AVANT结果小结DFS(至少3年随访期)–HRFOLFOX+BVZ1.17(0.98,1.39),(73%3yDFS)–HRXELOX+BVZ1.07(0.9,1.28),(75%3yDFS)–组间无差异–1年的DFSHR结果与NSABP-08类似,但1年后消失–是否rebound因素?•两组的复发部位类似(BVZ组并未更差)•复发后的生存时间差异不大SiteofRecurrence(ITTStageIII)FOLFOX4(N=955)n(%)FOLFOX4+Bev(N=960)n(%)XELOX+Bev(N=952)n(%)Patientswithtumorrecurrence*219(23)252(26)223(23)Localrecurrence39(4)42(4)47(5)Regionallymphnodes19(2)22(2)21(2)Distantlymphnodes36(4)31(3)30(3)Liver82(9)87(9)62(7)Lung45(5)63(7)57(6)Other62(6)88(9)64(7)1involvedsite164(17)192(20)177(19)>1involvedsite55(6)60(6)46(5)*Andwithoutevidenceofdiseaseatrandomization;percentagesbasedonN*Andwithoutevidenceofdiseaseatrandomization;percentagesbasedonNInterimOS(ITTStageIII)FOLFOX(N=955)FOLFOX4+Bev(N=960)XELOX+Bev(N=952)HR(95%CI)1.31(1.03,1.67)1.27(0.99,1.62)955960952914942920899925908884900894863869861844835840573573546FOLFOX4FOLFOX4+BevXELOX+BevNumberatrisk7767637654614494450.00.10.20.30.40.50.60.70.80.91.06183036424801224Time(months)54606672288269290010637064000Event-freerateFOLFOX4FOLFOX4+BevXELOX+BevNO147:FOLFIRI组数据伊立替康为基础的方案迄今未能显示用于CRC辅助化疗的价值–PETACC-3,ACCORD2,CALGB89803–3-yrDFS约60%–CALGB89803的分子生物学分析发现与KRAS基因状态无关N0147:西妥昔单抗+FOLFOX数据未能显示用于辅助治疗的益处NO147报告146例III期CRC接受FOLFIRI(106)和FOLFIRI+西妥昔单抗(40)的数据主要终点:DFS两组平衡好(KRASWT两组均为65%)N0147:2001年的初始研究设计RFOLFIRI5-FU400mg/m2+推注CPT-11180mg/m2+LV400mg/m2&5-FU2400mg/m2>46hq2w预计入组:3750例mFOLFOX65-FU400mg/m2+奥沙利铂85mg/m2+LV400mg/m2&5-FU2400mg/m2>46hq2wmFOLFOX6FOLFIRIHuangJ,etal.2011ASCOGIAbstract363.N0147:首次设计改变2004年9月添加西妥昔单抗–6组设计主要终点:两组KRAS突变型与野生型的DFS次要终点:OS,因加入西妥昔单抗而产生的毒性反应RFOLFIRI±西妥昔单抗FOLFOX±西妥昔单抗FOLFOXFOLFIRI±西妥昔单抗FOLFIRI±西妥昔单抗(C225)2011ASCO-GI报告Hu...