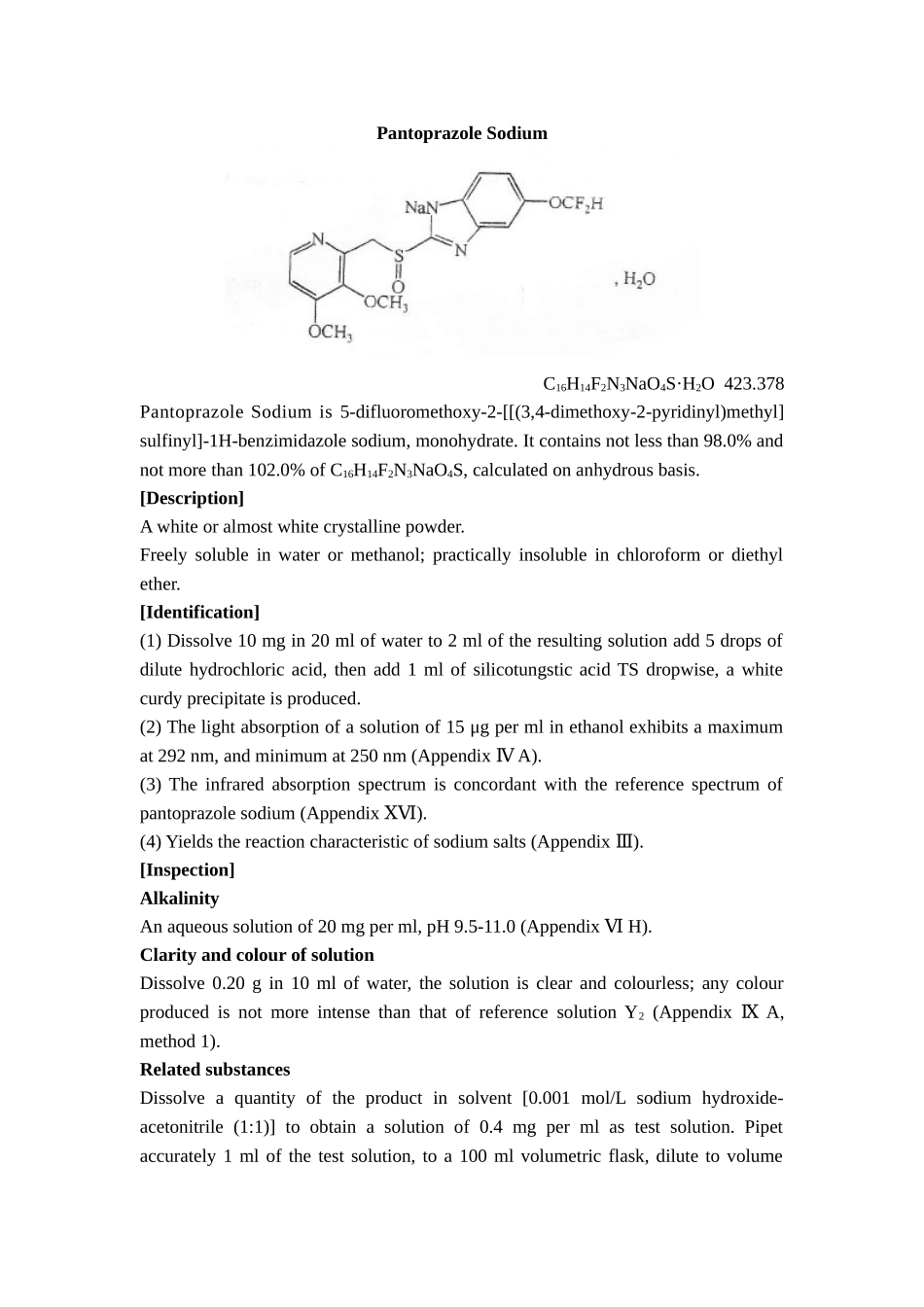
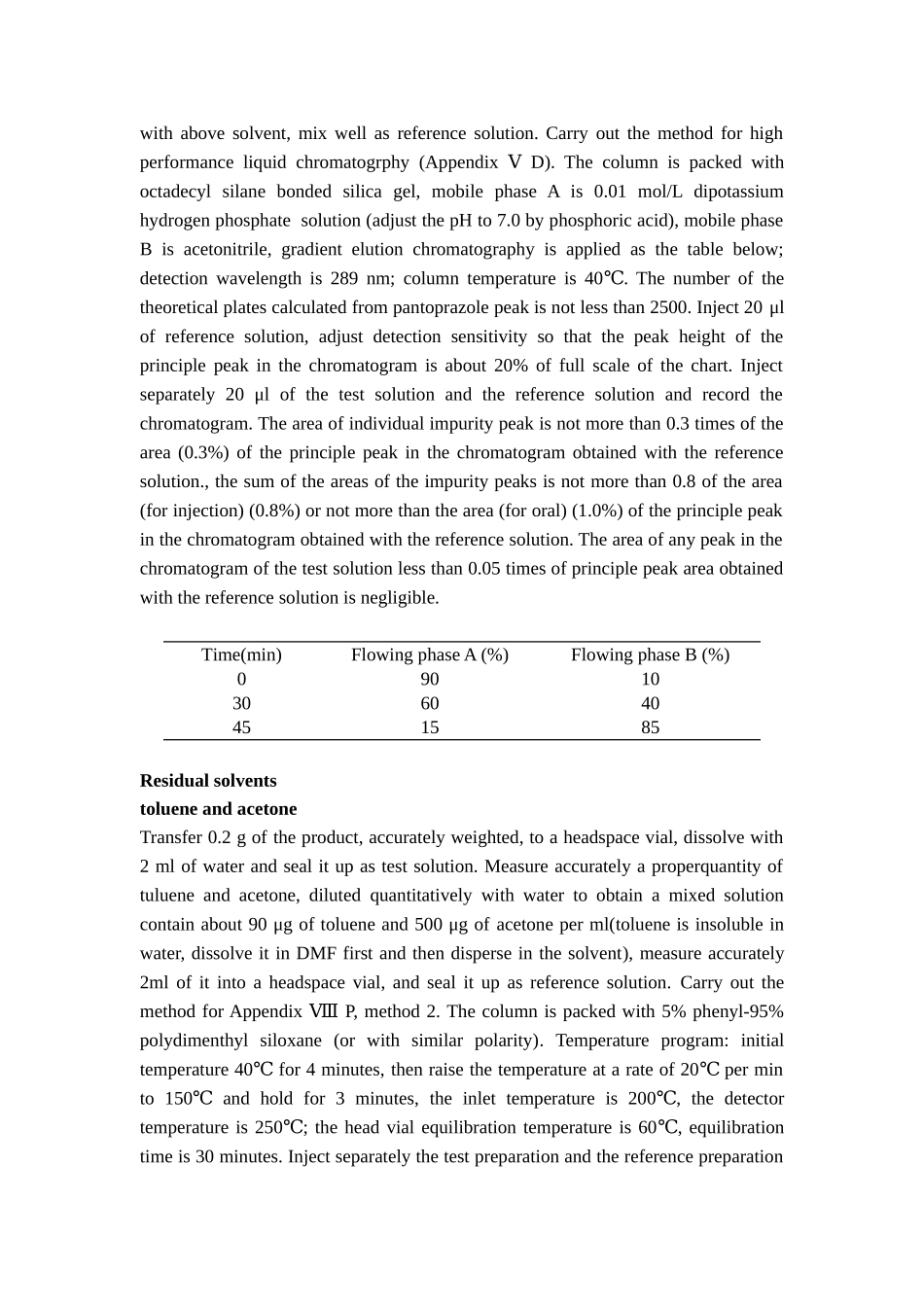
PantoprazoleSodiumC16H14F2N3NaO4S·H2O423.378PantoprazoleSodiumis5-difluoromethoxy-2-[[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazolesodium,monohydrate.Itcontainsnotlessthan98.0%andnotmorethan102.0%ofC16H14F2N3NaO4S,calculatedonanhydrousbasis.[Description]Awhiteoralmostwhitecrystallinepowder.Freelysolubleinwaterormethanol;practicallyinsolubleinchloroformordiethylether.[Identification](1)Dissolve10mgin20mlofwaterto2mloftheresultingsolutionadd5dropsofdilutehydrochloricacid,thenadd1mlofsilicotungsticacidTSdropwise,awhitecurdyprecipitateisproduced.(2)Thelightabsorptionofasolutionof15μgpermlinethanolexhibitsamaximumat292nm,andminimumat250nm(AppendixA).Ⅳ(3)Theinfraredabsorptionspectrumisconcordantwiththereferencespectrumofpantoprazolesodium(AppendixⅩⅥ).(4)Yieldsthereactioncharacteristicofsodiumsalts(Appendix).Ⅲ[Inspection]AlkalinityAnaqueoussolutionof20mgperml,pH9.5-11.0(AppendixⅥH).ClarityandcolourofsolutionDissolve0.20gin10mlofwater,thesolutionisclearandcolourless;anycolourproducedisnotmoreintensethanthatofreferencesolutionY2(AppendixⅨA,method1).RelatedsubstancesDissolveaquantityoftheproductinsolvent[0.001mol/Lsodiumhydroxide-acetonitrile(1:1)]toobtainasolutionof0.4mgpermlastestsolution.Pipetaccurately1mlofthetestsolution,toa100mlvolumetricflask,dilutetovolumewithabovesolvent,mixwellasreferencesolution.Carryoutthemethodforhighperformanceliquidchromatogrphy(AppendixⅤD).Thecolumnispackedwithoctadecylsilanebondedsilicagel,mobilephaseAis0.01mol/Ldipotassiumhydrogenphosphatesolution(adjustthepHto7.0byphosphoricacid),mobilephaseBisacetonitrile,gradientelutionchromatographyisappliedasthetablebelow;detectionwavelengthis289nm;columntemperatureis40℃.Thenumberofthetheoreticalplatescalculatedfrompantoprazolepeakisnotlessthan2500.Inject20μlofreferencesolution,adjustdetectionsensitivitysothatthepeakheightoftheprinciplepeakinthechromatogramisabout20%offullscaleofthechart.Injectseparately20μlofthetestsolutionandthereferencesolutionandrecordthechromatogram.Theareaofindividualimpuritypeakisnotmorethan0.3timesofthearea(0.3%)oftheprinciplepeakinthechromatogramobtainedwiththereferencesolution.,thesumoftheareasoftheimpuritypeaksisnotmorethan0.8ofthearea(forinjection)(0.8%)ornotmorethanthearea(fororal)(1.0%)oftheprinciplepeakinthechromatogramobtainedwiththereferencesolution.Theareaofanypeakinthechromatogramofthetestsolutionlessthan0.05timesofprinciplepeakareaobtainedwiththereferencesolutionisnegligible.Time(min)FlowingphaseA(%)FlowingphaseB(%)09010306040451585ResidualsolventstolueneandacetoneTransfer0.2goftheproduct,accuratelyweighted,toaheadspacevial,dissolvewith2mlofwaterandsealitupastestsolution.Measureaccuratelyaproperquantityoftulueneandacetone,dilutedquantitativelywithwatertoobtainamixedsolutioncontainabout90μgoftolueneand500μgofacetoneperml(tolueneisinsolubleinwater,dissolveitinDMFfirstandthendisperseinthesolvent),measureaccurately2mlofitintoaheadspacevial,andsealitupasreferencesolution.CarryoutthemethodforAppendixⅧP,method2.Thecolumnispackedwith5%phenyl-95%polydimenthylsiloxane(orwithsimilarpolarity).Temperatureprogram:initialtemperature40℃for4minutes,thenraisethetemperatureatarateof20permin℃to150andholdfor3minutes,℃theinlettemperatureis200℃,thedetectortemperatureis250℃;theheadvialequilibrationtemperatureis60℃,equilibrationtimeis30minutes.Injectseparately...