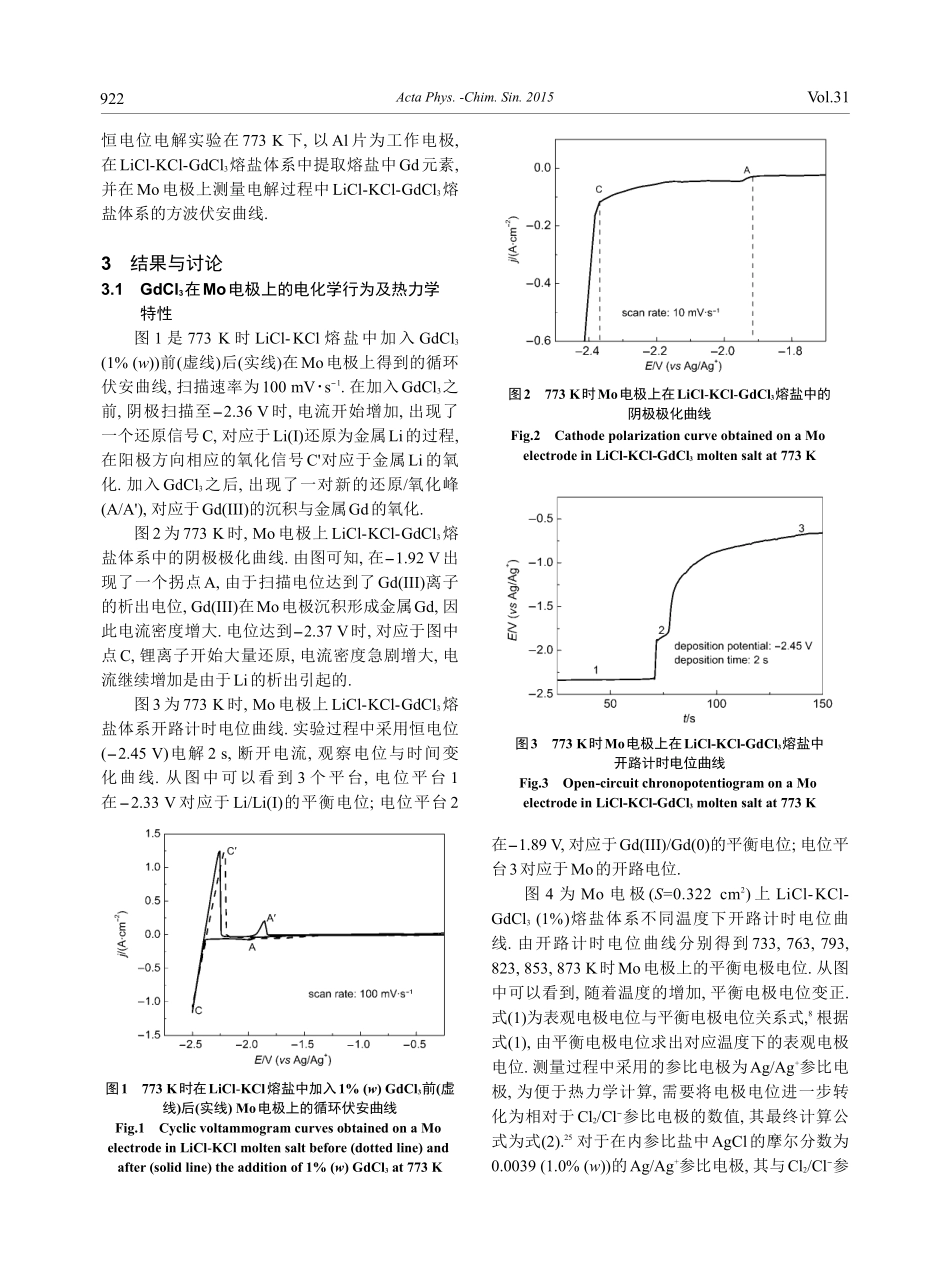
[Article]www.whxb.pku.edu.cn物理化学学报(WuliHuaxueXuebao)ActaPhys.-Chim.Sin.2015,31(5),920-926MayReceived:December18,2014;Revised:March24,2015;PublishedonWeb:March25,2015.∗Correspondingauthor.Email:y5d2006@hrbeu.edu.cn;Tel:+86-451-82569890.TheprojectwassupportedbytheNationalNaturalScienceFoundationofChina(21103033,21101040,91226201,51104050),SpecialFoundationofChinaandHeilongjiangPostdoctoralScienceFoundation,China(2013T60344,LBH-TZ0411),FundamentalResearchFundsfortheCentralUniversities,China(HEUCF20151007),andFoundationforUniversityKeyTeacherofHeilongjiangProvinceandHarbinEngineeringUniversity,China(1253G016,HEUCFQ1415).国家自然科学基金(21103033,21101040,91226201,51104050),中国和黑龙江博士后特别资助科学基金(2013T60344,LBH-TZ0411),中央高校基本科研业务经费(HEUCF20151007),黑龙江省普通高等学校和哈尔滨工程大学青年学术骨干支持计划项目(1253G016,HEUCFQ1415)资助©EditorialofficeofActaPhysico-ChimicaSinicadoi:10.3866/PKU.WHXB201503251氯化物熔盐体系中Gd的电化学行为及提取效率的评估杨晓南1颜永得1,2,*张密林1李星1薛云2韩伟1(1哈尔滨工程大学材料科学与化学工程学院,超轻材料与表面技术教育部重点实验室,哈尔滨150001;2哈尔滨工程大学核安全与仿真技术国防重点学科实验室,哈尔滨150001)摘要:研究了773K时GdCl3在LiCl-KCl熔盐体系中在Mo和Al电极上的电化学行为和热力学特性.利用开路计时电位曲线得到了Gd(III)/Gd(0)体系在723-873K温度范围的平衡电极电位和表观电极电位.结果表明:平衡电极电位和表观电极电位随着温度的升高而变正.通过吉布斯自由能变进一步计算得到了GdCl3在LiCl-KCl熔盐体系中不同温度下的活度系数.结合稳态极化曲线,求得去极化值,并通过热力学计算,求得773K时Gd的理论提取效率.在773K时,在LiCl-KCl-GdCl3体系中,以Al为工作电极在-1.5V左右通过恒电位电解提取Gd,电解20h后实际电解提取效率为94.22%.对电解沉积物进行X射线衍射(XRD)分析,检测到了Al3Gd合金相.关键词:熔盐;吉布斯自由能变;活度系数;提取效率;电解提取中图分类号:O646ElectrochemicalBehaviorandExtractionEfficiencyEvaluationofGdinChlorideMoltenSaltSystemYANGXiao-Nan1YANYong-De1,2,*ZHANGMi-Lin1LIXing1XUEYun2HANWei1(1KeyLaboratoryofSuperlightMaterialsandSurfaceTechnology,MinistryofEducation,CollegeofMaterialsScienceandChemicalEngineering,HarbinEngineeringUniversity,Harbin150001,P.R.China;2FundamentalScienceonNuclearSafetyandSimulationTechnologyLaboratory,HarbinEngineeringUniversity,Harbin150001,P.R.China)Abstract:TheelectrochemicalbehaviorandthermodynamicpropertiesofGdCl3inLiCl-KClmoltensaltsystemonbothMoandAlelectrodesat773Kwereinvestigated.AnopencircuitchronopotentiogramwasusedtodeterminetheequilibriumpotentialsandstandardapparentpotentialsoftheGd(III)/Gd(0)systeminthetemperaturerange723-873K.Theresultsshowedthattheequilibriumpotentialsandapparentstandardpotentialsbecamepositivewithincreasingtemperature.TheactivitycoefficientsofGdCl3werecalculatedatdifferenttemperatures.Thedepolarizationvalueswerecalculatedbysteadystatepolarizationtests,andthetheoreticalextractionefficiencywasobtainedat773K.PotentiostaticelectrolysiswasperformedtoextractgadoliniumfromLiCl-KCl-GdCl3moltensaltonAlelectrodesat-1.5V.Theextractionefficiencywasabout94.22%after20h.TheAl3GdphasewasidentifiedinthedepositbyX-raydiffraction(XRD).KeyWords:Moltensalt;Changeofgibbsfreeenergy;Activationcoefficient;Extractionefficiency;Electrolyticextraction920杨晓南等:氯化物熔盐体系中Gd的电化学行为及提取效率的评估No.51引言随着核能的广泛应用,乏燃料的处理已经成为制约核能可持续发展的关键问题之一.乏燃料是经过反应堆辐照后取出的核...