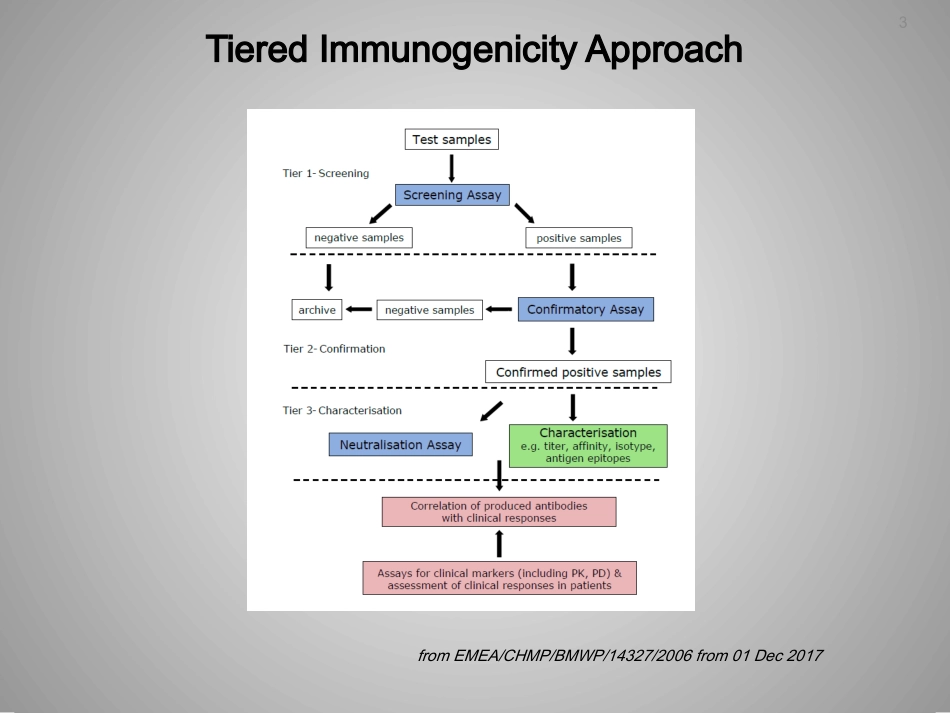
1ValidationofADAAssays–Recentexperiencesandissues2Whoiswho?3TieredImmunogenicityApproachfromEMEA/CHMP/BMWP/14327/2006from01Dec2017ChallengesinADAanalysisCutpointdetermination(Outliers,Pre-versusin-studycutpoints)Assaycontrols(includingNC,acceptancecriteria)Pre-existingantibodies(anti-CCD,anti-PEG)SensitivityScreeningassayConfirmatoryassay(spikingconcentration)DrugToleranceTargetTolerance5StandardApproachforADAValidationCutPointDetermination(Shankaretal)Data:~50drugnaïvesubjects,>=3runs(2analysts)InvestigateDistributionNon-normalNormalTransformdata(usuallylog)OutlierevaluationComparemeansandvariancesbetweenruns/instruments/analystsConfirmDistributionValidationcut-point(CP.V)Mean+1.645*SDorRobustalternativeNormal95thpercentileNon-normalCalculateCP.VandCFperinstrumentVariancesdifferentInstrumentorAnalystspecificfloatingCPDynamiccut-pointDetermineCPineachin-studyrunFixedcut-point=CP.VScreeningcut-pointVariancessimilarMeanssimilarMeansdifferentVariancesdifferentFixedcut-point(CP.V)perinstrumentDynamiccut-pointDetermineCPineachin-studyrunNC.V=Neg.ControlfromValidationrunsNC.IS=Neg.ControlfromIn-StudyrunVariancessimilarFloatingcut-pointNC.IS*(CP.V/NC.V),iflogNC.IS+(CP.V–NC.V),ifnotfromShankaretal,2008CutPointDetermination(Zhangetal)fromZhangetal,JIM,389(2013)79–87StepsforCPdeterminationSampledatanormalizationbydividingbytheaverageNCresponseonthesameplateS/NratioisobtainedforeachsampleineachrunLogarithmictransformationappliedtoallSNvaluesDetectandremovalofanalyticaloutliers(Grubbs’test)EliminateanddeletebiologicaloutliersbyBoxplotmethodwith3IQRNormalitytest(Shapiro-Wilks);parametricornonparametricmethodAnti-logtransformationtobeappliedtogetthefinalcutpoint.fromZhangetal,JIM,389(2013)79–87AnalyticalorBiologicalOutlier?SequentialOutlierElimination1.AnalyticalOutliersSubjectaverageddata2.BiologicalOutliersIdentify&excludeanalyticaloutliers(AO)fromeachassayrun/plateseparately.IterateuntilnomoreAOThenidentify&excludebiologicaloutliers(BO)byevaluatingthedistributionofsubjectaverageddata.IterateuntilnomoreBO.Thenverifydistributionofsubjectaverageddata.10Pre-existingAntibodiesEMA(2016):„Someindividual’s/patient’ssamplesmaycontainpre-existing(pre-treatment)antibodiesorpossiblyothersubstanceswhichproducesignificantpositiveresponsesinassays,andsoscreeningpatientsforthisisnecessarytoensurethatpost-treatmentdatacanbeinterpretedcorrectlyintermsoftreatmentemergentantibodies.”FDA(2016):Pre-existingantibodiesmayhaveclinicaleffectsandmayaffecttheefficacyofthetherapeuticproteinproductbeingtested.AnalternativetothequalitativescreeningassayapproachmaybeneededtoassessthequantityandqualityofADAwhenpre-existingantibodiesarepresent.Forexample,testingsamplesforanincreaseinADAusingasemi-quantitativeassaytypesuchasatiteringassaycanprovideinformationontheimpactofatherapeuticproteinproductonproductimmunogenicitythatisnotprovidedbyaqualitativeassay.EffectofnormalizedsignalsSuitabilityoftheNegativeControl•OftenafloatingCut-Pointisused(e.g.log(S/N))•However,normalizationisnotnecessarilybeneficial•Checksuitabilityofthenegativecontrolbyplottingthemeanresponseoftheindividualsamplesperrunagainstthemeanofthenegativecontrolofthesamerun•Normalizationisnotbeneficialiftherelationshiphaszeroornegativeslopevalue0.0080.010.0120.0140.0160.0180.020.0220.024Result123456Ru...