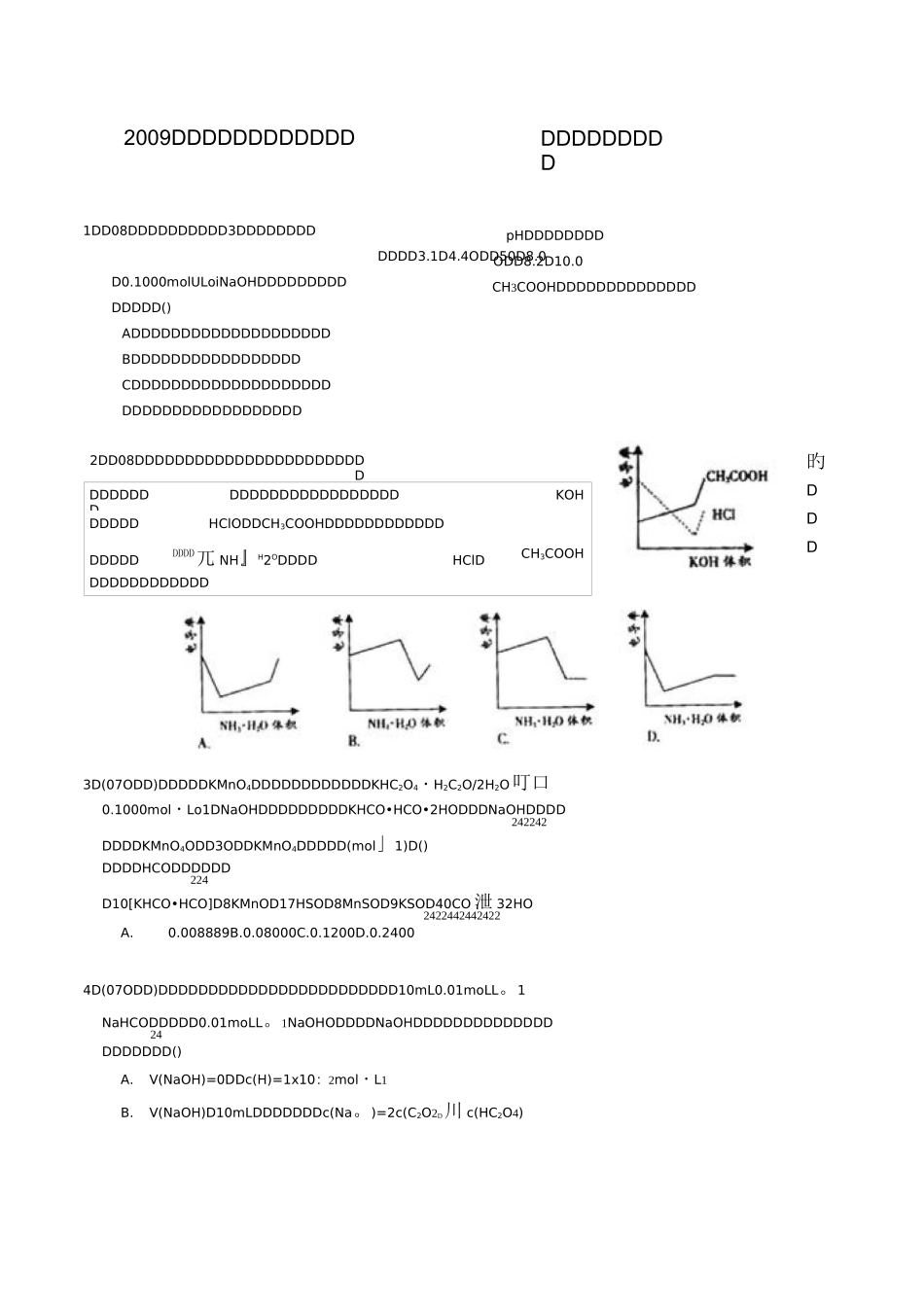
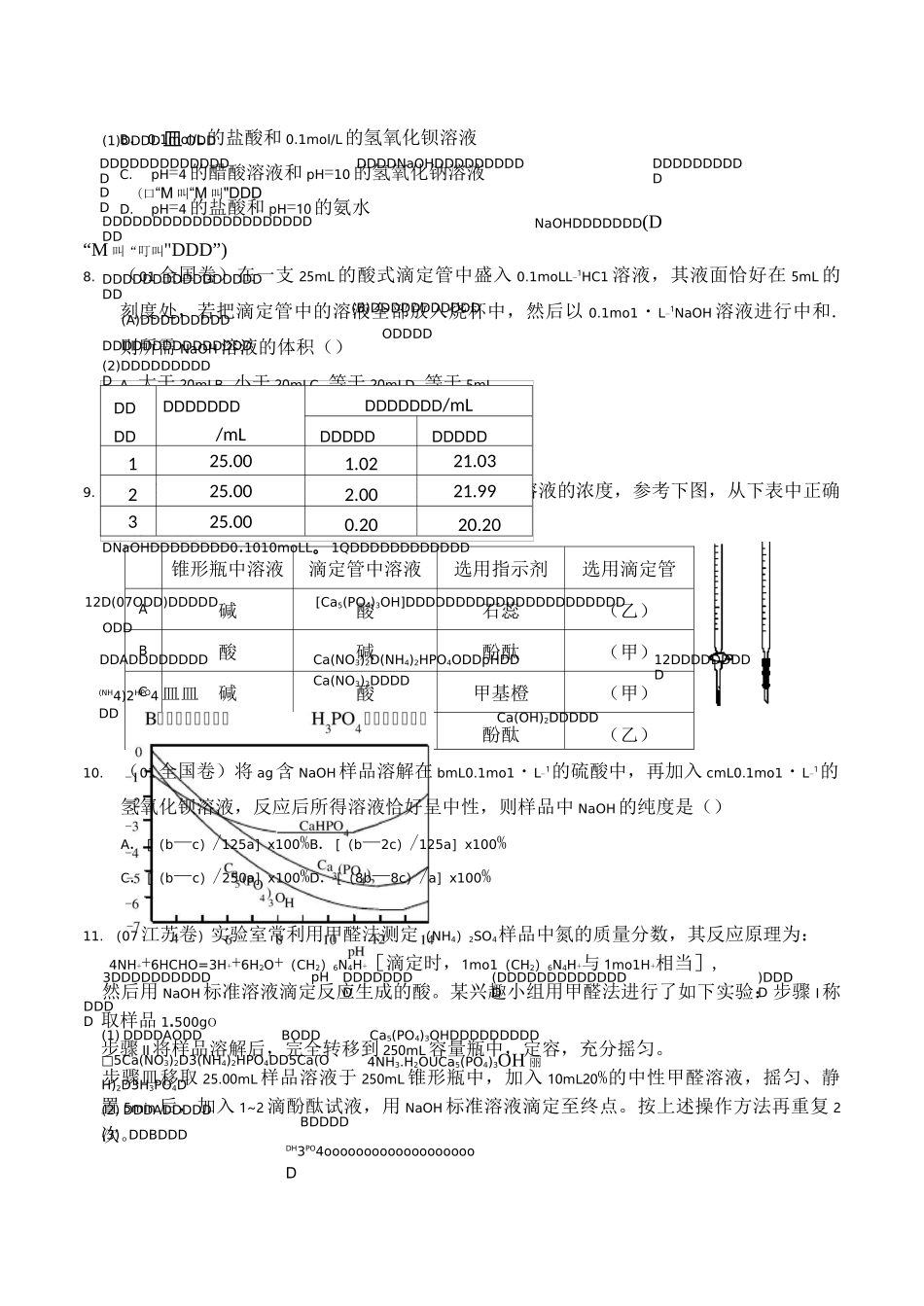
pHDDDDDDDDODD8.2D10.0CH3COOHDDDDDDDDDDDDDD1DD08DDDDDDDDDD3DDDDDDDDDDDD3.1D4.4ODD50D8.0D0.1000molULoiNaOHDDDDDDDDDDDDDD()ADDDDDDDDDDDDDDDDDDDDBDDDDDDDDDDDDDDDDDCDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDD3D(07ODD)DDDDDKMnO4DDDDDDDDDDDDKHC2O4・H2C2O/2H2O叮口0.1000mol・Lo1DNaOHDDDDDDDDDKHCO•HCO•2HODDDNaOHDDDD242242DDDDKMnO4ODD3ODDKMnO4DDDDD(mol」1)D()DDDDHCODDDDDD224D10[KHCO•HCO]D8KMnOD17HSOD8MnSOD9KSOD40CO 泄 32HO2422442442422A.0.008889B.0.08000C.0.1200D.0.24004D(07ODD)DDDDDDDDDDDDDDDDDDDDDDDD10mL0.01moLL。1NaHCODDDDD0.01moLL。1NaOHODDDDNaOHDDDDDDDDDDDDDD24DDDDDDD()A.V(NaOH)=0DDc(H)=1x10:2mol・L1B.V(NaOH)D10mLDDDDDDDc(Na。)=2c(C2O2D川 c(HC2O4)2009DDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDDKOHDDDDDHClODDCH3COOHDDDDDDDDDDDDDDDDDDDDD兀 NH』H2ODDDDHClDCH3COOHDDDDDDDDDDDD2DD08DDDDDDDDDDDDDDDDDDDDDDDD旳DDDC.V(NaOH)=10mL时,c(H+)=1x10-7mol・L-1D.V(NaOH)>10mL时,c(Na+)>c(C2O2-)>c(HC2O-)5.(08山东卷)某温度时,BaSO4在水中的沉淀溶解平衡曲线如图所示。下列说法正确的是()提示:BaSO4(s)=Ba2+(aq)+SO2-4(aq)的平衡常数 Kap=c(Ba2+).c(SO2-4),称为溶度积常数。A.加入 Na2SO4可以使溶液由 a点变到 b点B.通过蒸发可以使溶液由 d点变到 c点C.d点无 BaSO4沉淀生成D.a点对应的 K大于 c点对应的 Kapap6.(06北京卷)某酸 HX稀溶液和某碱 YOH稀溶液的物质的量浓度相等,两溶液混合后,溶液的pH大于7。下表中判断合理的是()编号HXYOH溶液的体积关系①强酸强碱V(HX)=V(YOH)②强酸强碱V(HX)VV(YOH)③强酸弱碱V(HX)=V(YOH)④弱酸强碱V(HX)=V(YOH)A.①③B.②③C.①④D.②④7.(06上海卷)室温下,下列溶液等体积混合后,所得溶液的 pH一定大于 7的是(A.0.1mol/L的盐酸和 0.1mol/L的氢氧化钠溶液锥形瓶中溶液滴定管中溶液选用指示剂选用滴定管A碱酸石蕊(乙)B酸碱酚酞(甲)C碱酸甲基橙(甲)D酸碱酚酞(乙)B.0.1mol/L的盐酸和 0.1mol/L的氢氧化钡溶液C.pH=4的醋酸溶液和 pH=10的氢氧化钠溶液D.pH=4的盐酸和 pH=10的氨水8.(01 全国卷)在一支 25mL 的酸式滴定管中盛入 0.1moLL-1HC1 溶液,其液面恰好在 5mL 的刻度处,若把滴定管中的溶液全部放入烧杯中,然后以 0.1mo1・L-1NaOH 溶液进行中和.则所需 NaOH溶液的体积()A.大于 20mLB.小于 20mLC.等于 20m...