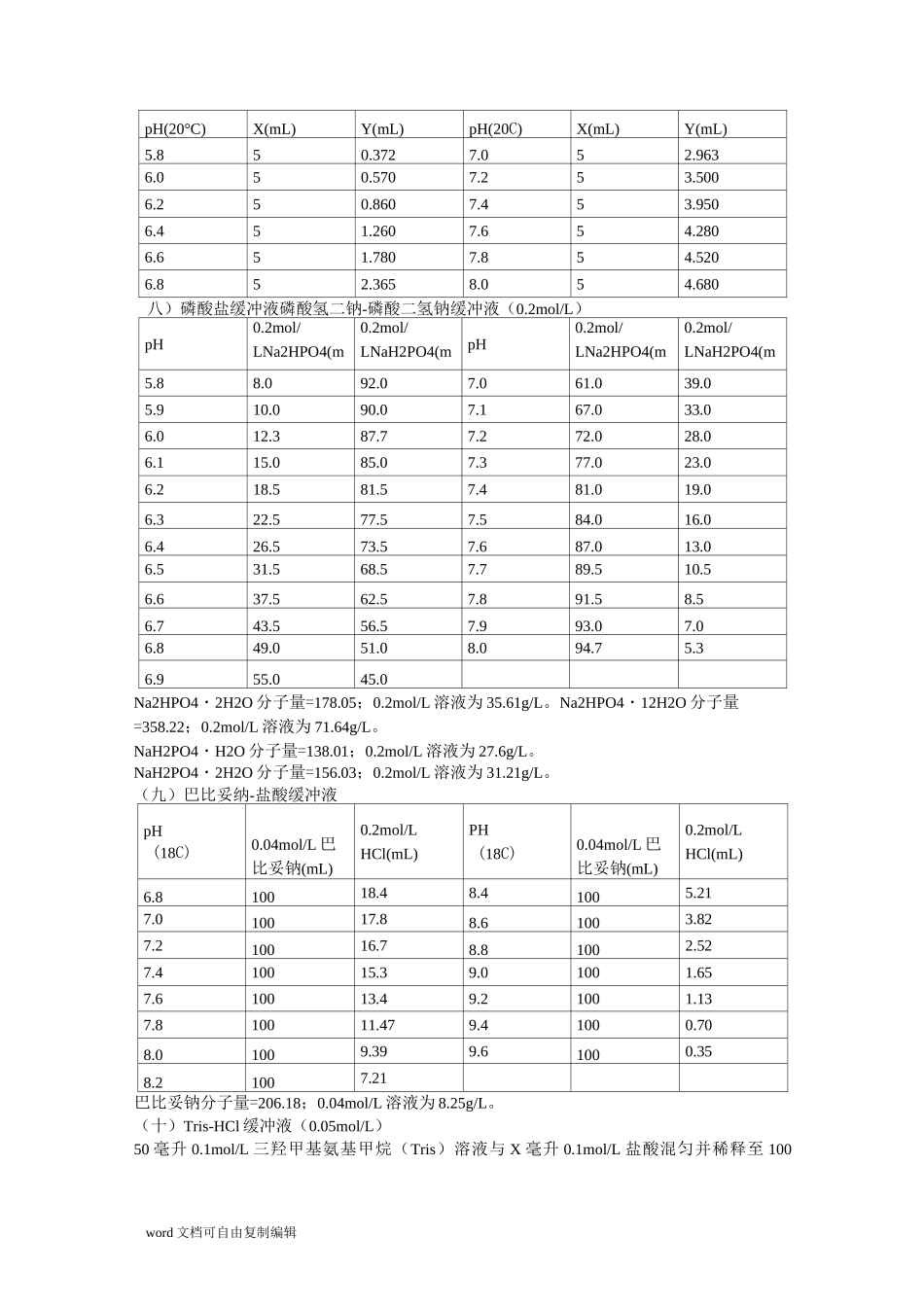
word 文档可自由复制编辑不同缓冲液的缓冲范围pH缓冲液六十一秒的常用缓冲溶液的配制&缓冲溶液原理(2007 年 6 月 16 日更新)(一)甘氨酸-盐酸缓冲液(0.05mol/L)X 毫升 0.2mol/L 甘氨酸+Y 毫升 0.2mol/LHC1,再加水稀释至 200 毫升。pHXYpHXY2.25044.03.05011.42.45032.43.2508.22.65024.23.4506.42.85016.83.6505.0甘氨酸分子量=75.07。0.2mol/L 甘氨酸溶液含 15.01g/L。(二)邻苯二甲酸-盐酸缓冲液(0.05mol/L)X 毫升 0.2mol/L 邻苯二甲酸氢钾+Y 毫升 0.2mol/LHCl,再加水稀释至 20 毫升。pH(20°C)XYpH(20C)XY2.254.6703.251.4702.453.9603.450.9902.653.2952.650.5972.852.6423.850.2633.052.032邻苯二甲酸氢钾分子量=204.23。0.2mol/L 邻苯二甲酸氢钾溶液含 40.85g/L。三)磷酸氢二钠-柠檬酸缓冲液pH0.2mol/LNa2HPO4(mL)0.1mol/L 柠檬酸(mL)pH0.2mol/LNa2HPO4(mL)0.1mol/L 柠檬酸(mL)2.20.4019.605.210.729.282.41.2418.765.411.158.852.62.1817.825.611.608.402.83.1716.835.812.097.913.04.1115.896.012.637.373.24.9415.066.213.226.783.45.7014.306.413.856.153.66.4413.566.614.555.453.87.1012.906.815.454.554.07.7112.297.016.473.534.28.2811.727.217.392.614.48.8211.187.418.171.834.69.3510.657.618.731.274.89.8610.147.819.150.855.010.309.708.019.450.55Na2HPO4 分子量=141.98;0.2mol/L 溶液为 28.40g/L。Na2HPO4・2H2O 分子量=178.05;0.2mol/L 溶液为 35.61g/L。Na2HPO4・12H2O 分子量=358.22;0.2mol/L 溶液为 71.64g/L。word 文档可自由复制编辑C6H8O7・H2O 分子量=210.14;0.1mol/L 溶液为 21.01g/L。(四)柠檬酸-氢氧化钠-盐酸缓冲液pH钠离子浓度(mol/L)柠檬酸(g)C6H8O7・H2O氢氧化钠(g)NaOH97%盐酸(mL)HCl(浓)最终体积(L)①2.20.2021084160103.10.2021083116103.30.2021083106104.30.202108345105.30.3524514468105.80.45285186105106.50.3826615612610① 使用时可以每升中加入 1 克酚,若最后 pH 值有变化,再用少量 50%氢氧化钠溶液或浓盐酸调节,冰箱保存。五)柠檬酸-柠檬酸钠缓冲液(0.1mol/L)pH0.1mol/L 柠檬酸(mL)0.1mol/L柠檬酸钠(mL)pH0.1mol/L 柠檬酸(mL)0.1mol/L柠檬酸钠(mL)3.018.61.45.08.211.83.217.22.85.27.312.73.416.04.05.46.413.63.614.95.15.65.514.53.814.06.05.84.715.34.013.16.96.03.816.24.212.37.76.22.817.24.411.48.66.42.018.04.6...