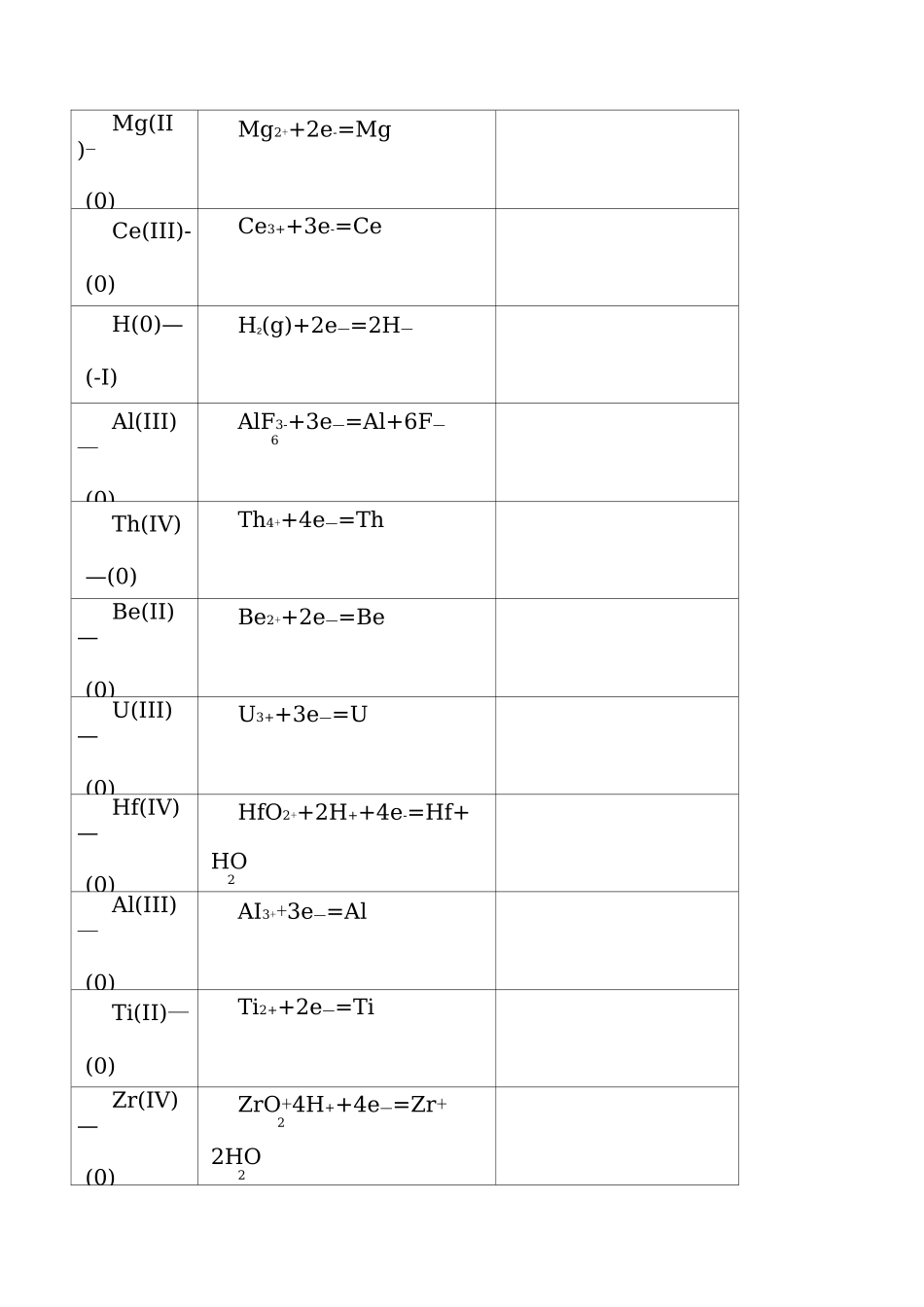
--标准电极电势表1在酸性溶液中(298K)电对方程式E/VLi⑴—(0)Li++e—=LiCs⑴—(0)Cs++e—=CsRb(I)_(0)Rb++e—=RbK(I)—(0)K++e—=KBa(II)—(0)Ba2+2eBaSr(II)—(0)Sr2++2e—=SrCa(II)—(0)Ca2+2eCaNa(I)_(0)Na++e—=NaLa(III)—(0)La3++3e—=LaMg(II)-(0)Mg2++2e-=MgCe(III)-(0)Ce3++3e-=CeH(0)—(-I)H2(g)+2e—=2H—Al(III)—(0)AlF3-+3e—=Al+6F—6Th(IV)—(0)Th4++4e—=ThBe(II)—(0)Be2++2e—=BeU(III)—(0)U3++3e—=UHf(IV)—(0)HfO2++2H++4e-=Hf+HO2Al(III)—(0)AI3++3e—=AlTi(II)—(0)Ti2++2e—=TiZr(IV)—(0)ZrO+4H++4e—=Zr+22HO2Si(IV)-(0)[SiF]2-+4e-=Si+6F-6Mn(II)-(0)Mn2++2e-=MnCr(II)-(0)Cr2++2e—=CrTi(III)—(II)Ti3++e—=Ti2+B(III)—(0)HBO+3H++3e—=B+333HO2*Ti(IV)—(0)TiO+4H++4e—=Ti+22HO2Te(0)—(—II)Te+2H++2e—=HTe2Zn(II)—(0)Zn2++2e—=ZnTa(V)—(0)TaO+10H++10e—=2Ta+255HO2Cr(III)—(0)Cr3++3e—=CrNb(V)—(0)NbO+10H++10e—=2Nb+255HO2As(O)—(—III)As+3H++3e—=AsH3U(IV)—(III)U4++e—=U3+Ga(III)—(0)Ga3++3e—=GaP(I)—(0)HPO+H++e—=P+322HO2P(III)—(I)HPO+2H++2e—=33HPO+HO322*C(IV)—(III)2CO+2H++2e—=HCO2224Fe(II)—(0)Fe2++2e—=FeCr(III)—(II)Cr3++e—=Cr2+Cd(II)—(0)Cd2++2e—=CdSe(0)—(—II)Se+2H++2e—=^Se(aq)Pb(II)—(0)PbI+2e—=Pb+2I2Eu(III)-(II)Eu3++e—=Eu2+Pb(II)—(0)PbSO+2e—=Pb+SO2—44In(III)—(0)In3++3e—=InTl(I)—(0)Tl++e—=TlCo(II)—(0)C02++2e-=CoP(V)—(III)HPO+2H++2e—=34HPO+HO332Pb(II)—(0)PbCl+2e—=Pb+2C1—2Ni(II)—(0)Ni2++2e-=NiV(III)—(II)V3++e—=V2+Ge(IV)—(0)HGeO+4H++4e—=Ge23+3HO2Ag(I)—(0)AgI+e-=Ag+I-Sn(II)-(0)Sn2++2e-=SnPb(II)-(0)Pb2++2e-=Pb*C(IV)-(II)CO(g)+2H++2e-=CO+HO2P(0)-(-III)P(white)+3H++3e-=PH3(g)3Hg(I)-(0)Hgzi+2e-=2Hg+2I-Fe(III)-(0)Fe3++3e-=FeH(I)-(0)2H++2e—=H2Ag(I)-(0)AgBr+e-=Ag+Br-S-(II)SO2-+2e-=2SO24623*Ti(IV)-(III)TiO2++2H++e-=Ti3++HO2S(0)-(-II)S+2H++2e-=^S(aq)Sn(IV)-Sn4++2e-=Sn2+(II)Sb(III)—(0)SbO+6H++6e—=2Sb+233HO2Cu(II)—⑴Cu2++e—=Cu+Bi(III)—(0)BiOCl+2H++3e—=Bi+Cl-+HO2S(VI)—(IV)SO2—+4H++2e—=HSO423+HO2Sb(III)—(0)SbO++2H++3e-=Sb+HO2Ag⑴—(0)AgCl+e-=Ag+Cl—As(III)—(0)HAsO+3H++3e—=As+22HO2Hg(I)—(0)H^C^+2e—=2Hg+2Cl—(饱和KCl)Bi(III)—(0)BiO++2H++3e—=Bi+HO2U(VI)—(IV)UO2++4H++2e-=U4++22HO2C(IV)—2HCNO+2H++2e—=(CN)?(III)+2HO2V(IV)-VO2++2H++e—=V3++(III)HO2Cu(II)—Cu2++2e—=Cu(0)Re(VII)ReO—+8H++7e—=Re+4—(0)4HO2Ag⑴—AgCrO+2e—=2Ag+(0)CrO2—4SQV)—HSO+4H++4e—=S+23(0)3HO2Cu(I)—Cu++e—=Cu(0)I(0)—(—I+2e—=2I—2I)I(0)—(—I-+2e—=3I—3I)As(V)—HAsO+2H++2e—=34(III)HAsO+2HO22Sb(V)—SbO+6H++4e—=2SbO++25(III)3HO2Te(IV)—TeO+4H++4e—=Te+2(0)2HO2U(V)-(IV)UO++4H++e—=U4++22HO2**Hg(II)-(I)2HgCl+2e-=HgCl+2Cl匕2匕22Pt(IV)-(II)[PtCl]2-+2e-=[PtCl]2-+642Cl-O(0)-(-I)O+2H++2e—=HO222Pt(II)-(0)[PtCl]2-+2e-=Pt+4Cl-4*Se(IV)-(0)HSeO+4H++4e-=Se+233HO2Fe(III)-(II)Fe3++e-=Fe2+Hg(I)-(0)H^2++2e-=2HgAg(I)-(0)Ag++e-=AgOs(VIII)-(0)OsO+8H++8e—=Os+44HO2N(V)-2NO-+4H++2e-=NO324(IV)+2HO2Hg(II)-(0)Hg2++2e-=HgSi(IV)-(0)(quartz)SiO+4H++4e-=Si+2HOCu(II)—⑴Cu2++I-+e-=CuIN(III)—(I)2HNO+4H++4e—=2HNO+2HO2222Hg(II)—(I)2Hg2++2e-=Hg2+N(V)—(III)NO—+3H++2e—=HNO32+HO2Pd(II)—(0)Pd2++2e-=PdN(V)—(II)NO—+4H++3e—=NO+32HO2N(III)—(II)HNO+H++e—=NO+2HO2I(I)—(—I)HIO+H++2e—=I-+HO2V(V)—VO++2H++e—=VO2++2(IV)HO2V(V)-(IV)V(OH)++2H++e—=VO2++43HO2Au(III)-(0)[AuCl]—+3e—=Au+4Cl—4Te(VI)—(IV)HTeO+2H++2e—=TeO+6624HO2N(IV)—(II)NO+4H++4e—=2NO+242HO2N(IV)—(III)NO+2H++2e—=2HNO242I(V)—(—I)IO—+6H++6e—=I—+33HO2Br(0)—(—I)Br(aq)+2e—=2Br—Se(VI)—(IV)SeO2—+4H++2e—=4HSeO+HO232ci(V)—(IV)ClO—+2H++e—...