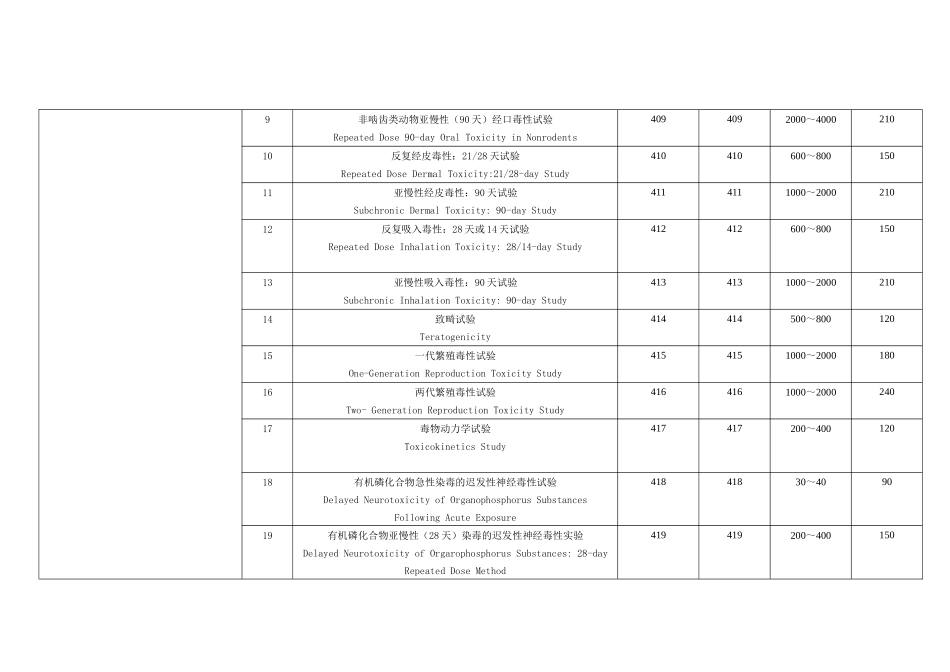
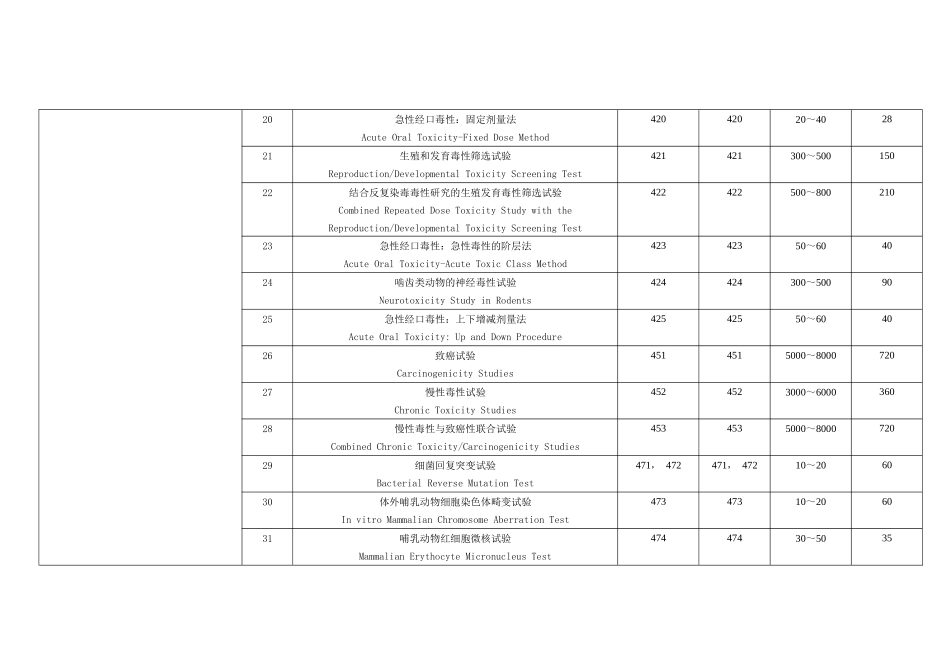
北京协和建昊医药技术开发有限责任公司Beijing Uuion-Genius Pharmaceutical Technology Ltd 项目领域Testing field序号No.项目名称Name of study方法号Method codeOECD 编号OECD code所需样品量(克)Needed sample(g)所需时间(天)Needed Time(d)健康毒理Health Effects1急性经口毒性试验Acute Oral Toxicity Test40140120~30282急性经皮毒性试验Acute Dermal Toxicity Test40240210~20283急性吸入毒性试验Acute Inhalation Toxicity Test40340320~30284急性皮肤刺激性/腐蚀性试验Acute Dermal Irritation/Corrosion Test40440410~20285急性眼刺激性/腐蚀性试验Acute Eye Irritation/Corrosion Test40540510~20286皮肤致敏试验Skin Sensitization Test40640620~30407啮齿类动物 28 天反复经口毒性试验Repeated Dose 28-day Oral Toxicity Study in Rodents407407600~8001508啮齿类动物亚慢性(90 天)经口毒性试验Repeated Dose 90-day Oral Toxicity in Rodents4084081000~20002109非啮齿类动物亚慢性(90 天)经口毒性试验Repeated Dose 90-day Oral Toxicity in Nonrodents4094092000~400021010反复经皮毒性:21/28 天试验Repeated Dose Dermal Toxicity:21/28-day Study410410600~80015011亚慢性经皮毒性:90 天试验Subchronic Dermal Toxicity: 90-day Study4114111000~200021012反复吸入毒性:28 天或 14 天试验Repeated Dose Inhalation Toxicity: 28/14-day Study412412600~80015013亚慢性吸入毒性:90 天试验Subchronic Inhalation Toxicity: 90-day Study4134131000~200021014致畸试验Teratogenicity414414500~80012015一代繁殖毒性试验One-Generation Reproduction Toxicity Study4154151000~200018016两代繁殖毒性试验Two- Generation Reproduction Toxicity Study4164161000~200024017毒物动力学试验Toxicokinetics Study417417200~40012018有机磷化合物急性染毒的迟发性神经毒性试验Delayed Neurotoxicity of Organophosphorus Substances Following Acute Exposure41841830~409019有机磷化合物亚慢性(28 天)染毒的迟发性神经毒性实验Delayed Neurotoxicity of Orgarophosphorus Substances: 28-day Repeated Dose Method419419200~40015020急性经口毒性:固定剂量法Acute Oral Toxicity-Fixed D...