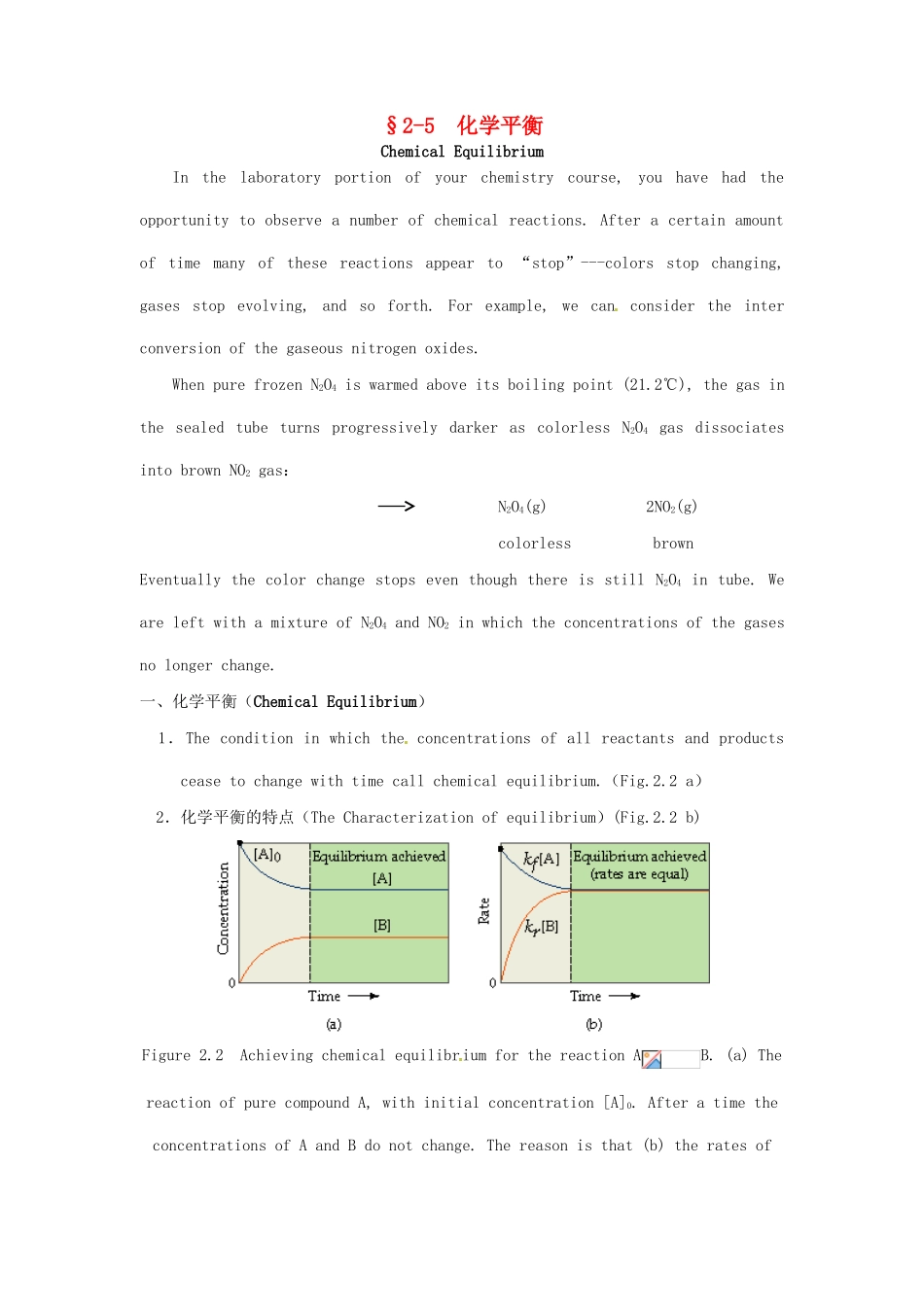
§2-5 化学平衡Chemical EquilibriumIn the laboratory portion of your chemistry course, you have had the opportunity to observe a number of chemical reactions. After a certain amount of time many of these reactions appear to “stop”---colors stop changing, gases stop evolving, and so forth. For example, we can consider the inter conversion of the gaseous nitrogen oxides.When pure frozen N2O4 is warmed above its boiling point (21.2℃), the gas in the sealed tube turns progressively darker as colorless N2O4 gas dissociates into brown NO2 gas: N2O4(g) 2NO2(g) colorless brownEventually the color change stops even though there is still N2O4 in tube. We are left with a mixture of N2O4 and NO2 in which the concentrations of the gases no longer change.一、化学平衡(Chemical Equilibrium) 1.The condition in which the concentrations of all reactants and products cease to change with time call chemical equilibrium.(Fig.2.2 a) 2.化学平衡的特点(The Characterization of equilibrium)(Fig.2.2 b)Figure 2.2 Achieving chemical equilibrium for the reaction AB. (a) The reaction of pure compound A, with initial concentration [A]0. After a time the concentrations of A and B do not change. The reason is that (b) the rates of the forward reaction (kf[A]) and the reverse reaction (kr[B]) become equal. Chemical equilibrium occurs when opposing reactions are proceeding at equal rates: Forward reaction:A B rate = k f [A],Reverse reaction:B A rate = k r [B]。 At equilibrium:kf [A] = kr [B],rearranging this equation gives. constant3.化学平衡表达式(The general equilibrium equation) 对于化学反应 A(g) + B(g) C(g) + D(g) 而言 (1) 假设上述反应为基元反应,则平衡时, ∴constant (T≡c) (2) 假设上述反应分两步进行,中间产物为 Q如: A(g) + A...