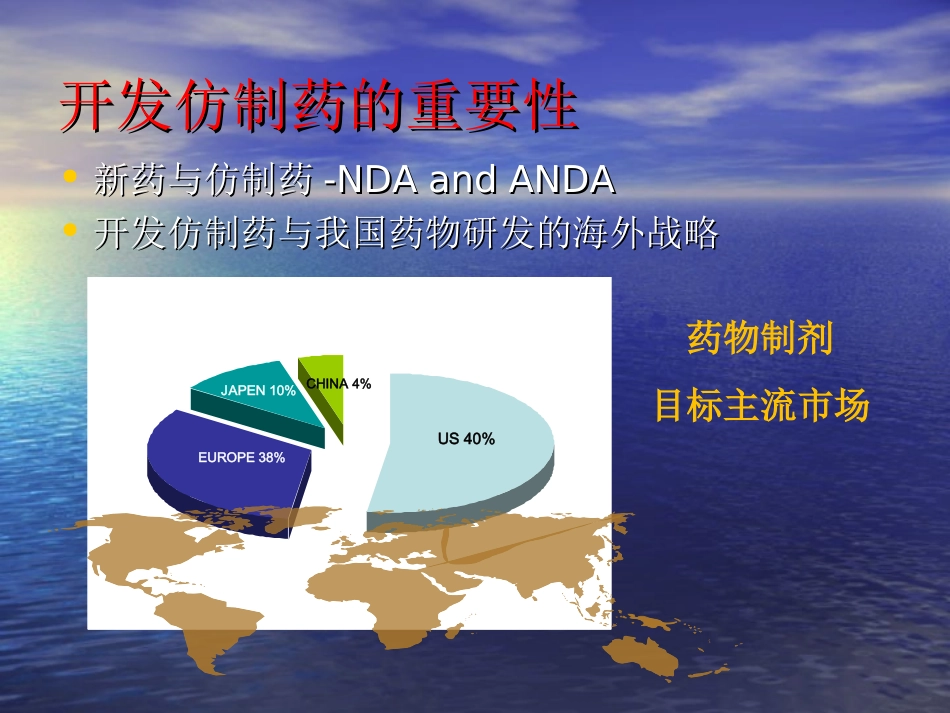
开发报批美国FDA的仿制药与相关问题探讨上海复星普适医药科技有限公司上海复星普适医药科技有限公司何平何平内容提要内容提要•开发仿制药的重要性和机遇开发仿制药的重要性和机遇•开发仿制药的挑战开发仿制药的挑战•申报仿制药的分类申报仿制药的分类•仿制药研发团队仿制药研发团队•仿制药的研发过程仿制药的研发过程•QbDQbD在制剂开发中怎么体现在制剂开发中怎么体现•研发研发((高难高难))仿制药的一些体会仿制药的一些体会::案例研究案例研究开发仿制药的重要性开发仿制药的重要性•新药与仿制药新药与仿制药-NDA-NDAandandANDAANDA•开发仿制药与我国药物研发的海外战略开发仿制药与我国药物研发的海外战略US40%EUROPE38%JAPEN10%CHINA4%US40%EUROPE38%JAPEN10%CHINA4%药物制剂目标主流市场开发仿制药的挑战性开发仿制药的挑战性开发仿制药更具挑战性开发仿制药更具挑战性–药物制剂药物制剂–专利专利–仿制药的竞争仿制药的竞争•仿制药厂之间的竞争仿制药厂之间的竞争•由品牌药转成仿制药由品牌药转成仿制药仿制药竞争的方式仿制药竞争的方式HOWTOCOMPETEHOWTOCOMPETE•CostCost-IRProduct-IRProduct–RawMaterialsRawMaterials–ProcessProcess–FinishedProductFinishedProduct•TechnologyTechnology-ModifiedRelease-ModifiedReleaseProductsProducts申报申报((仿制仿制))新药的分类新药的分类规范市场规范市场(FDA)(FDA)11。。P-IP-I22。。P-IIP-II33。。P-IIIP-III44。。P-IVP-IV(1(1ststtofile)tofile)中国市场(中国市场(sFDsFDAA))11类类22类类33类类44类类55类类66类类仿制药研发团队CONCEPT-1BUILDUPATEAMINFORMATIONFORMULATIONPRODUCTREGULATORYANALYTICALBIO-PHARMACEUTICALPROJECTLEGEL•DRUGDELIVERYSYSTEMSFORORALDRUGDELIVERYSYSTEMSFORORALSOLIDFORMULATIONS-MRSOLIDFORMULATIONS-MR–MATRIXSYSTEMSMATRIXSYSTEMS–RESERVIORSYSTEMSRESERVIORSYSTEMS–OSMOTICALPUMPSYSTEMSOSMOTICALPUMPSYSTEMS–COMBO-SYSTEMSCOMBO-SYSTEMS缓控释给药的技术平台和给药系统缓控释给药的技术平台和给药系统CONCEPT-2BUILDUPACONCEPT-2BUILDUPASYSTEMSYSTEMProductDevelopmentRoadmap仿制药的研发过程•Quality–Acceptablylowriskoffailingtoachievethedesiredclinicalattributes•PharmaceuticalQuality=f{drugsubstance,excipients,manufacturing..}•QbD–‘Productandprocessperformancecharacteristicsscientificallydesignedtomeetspecificobjectives,notmerelyempiricallyderivedfromperformanceoftestbatches’WhatisQbD(QualitybyQualitybyDesign)Design)?QbD在制剂开发中怎么体现?WhatisQbD?QbD在制剂开发中怎么体现?•PharmaceuticalQualitybyDesignPharmaceuticalQualitybyDesign(QbD)(QbD)–QbDmeansdesigninganddevelopingQbDmeansdesigninganddevelopingformulationsandmanufacturingformulationsandmanufacturingprocessestoensurepredefinedproductprocessestoensurepredefinedproductqualityquality•UnderstandingandcontrollingformulationUnderstandingandcontrollingformulationandmanufacturingprocessvariablesandmanufacturingprocessvariablesaffectingthequalityofadrugproductaffectingthequalityofadrugproductEssentialelementsofQbDDefinitionofthequalitytargetproductprofileHighlevelqualityaspectsoftheproduct:purity,drugrelease(dissolution/disintegrationtime),pharmacokineticprofile,etc.Criticalqualityattributes(CQAs)fordrugproduct•CharacteristicsofDPwhichhaveimpactondesiredprofile•ConsciousattempttostudyandcontrolCriticalProcessParameters(CPPs)•IdentificationofmaterialpropertiesandprocessparameterswhichhaveeffectonproductCQAsDesignSpace:ThemultidimensionalcombinationandinteractionofinputvariablesandprocessparametersthathavebeendemonstratedtoprovideassuranceofqualityIdentificationofacontrolstrategyforcriticalprocessparametersWhatisQbD?QbD在制剂开发中...