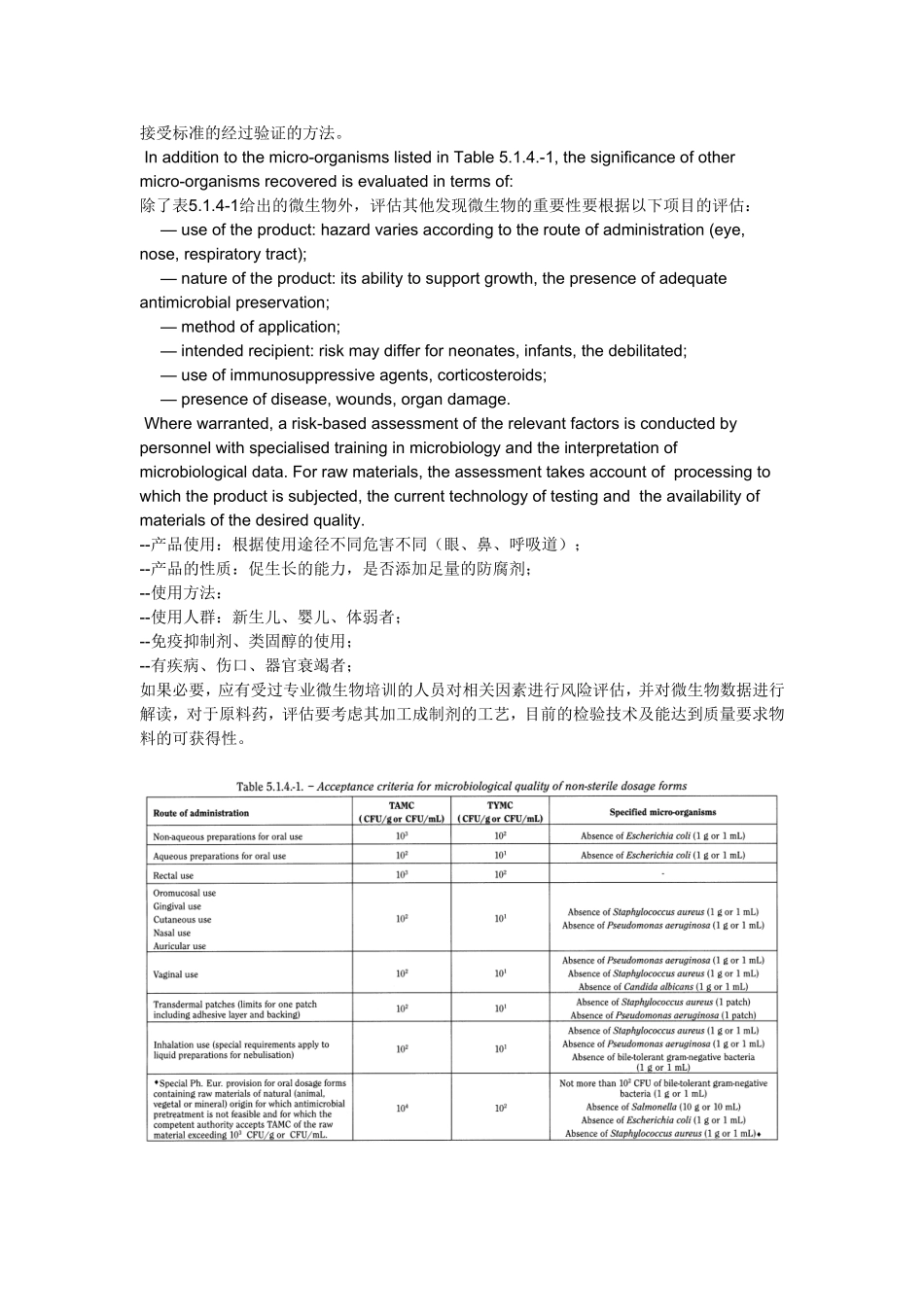
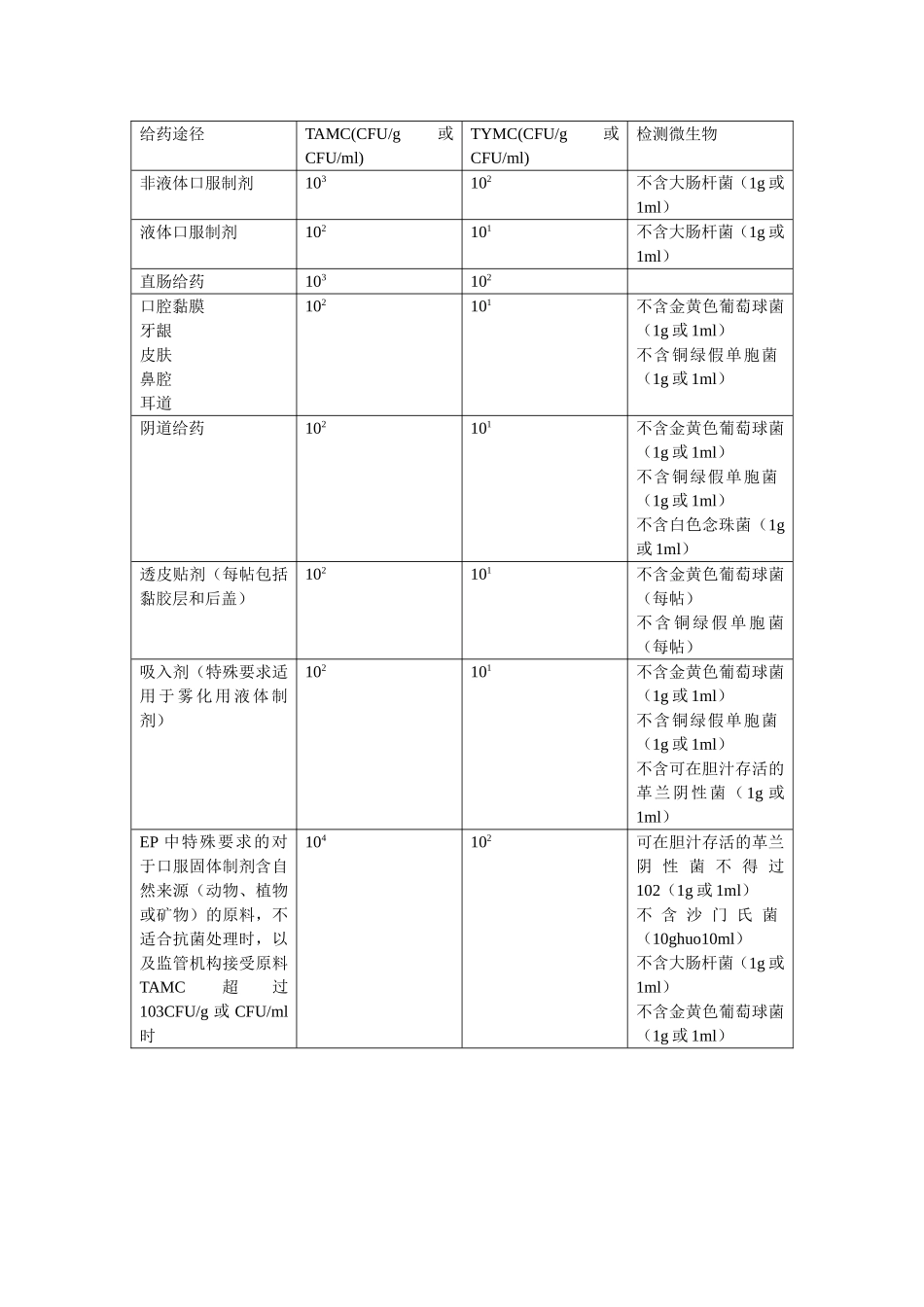
AppendixXVID.MicrobiologicalQualityofNon-sterilePharmaceuticalPreparationsandSubstances非无菌制剂的微生物限度(Ph.Eur.generaltext5.1.4)Thepresenceofcertainmicro-organismsinnon-sterilepreparationsmayhavethepotentialtoreduceoreveninactivatethetherapeuticactivityoftheproductandhasapotentialtoadverselyaffectthehealthofthepatient.ManufacturersthereforehavetoensurealowbioburdenoffinisheddosageformsbyimplementingcurrentguidelinesonGoodManufacturingPracticeduringthemanufacture,storageanddistributionofpharmaceuticalpreparations.非无菌制剂中存在一定量的微生物可能导致药物的治疗活性降低或消失,并对患者健康有着潜在的危害。因此生产商应在药品生产、储存、运输过程中实施GMP管理,使制剂的微生物负荷保持在低水平。Microbialexaminationofnon-sterileproductsisperformedaccordingtothemethodsgiveningeneralchapters2.6.12and2.6.13.Acceptancecriteriafornon-sterilepharmaceuticalproductsbaseduponthetotalaerobicmicrobialcount(TAMC)andthetotalcombinedyeasts/mouldscount(TYMC)aregiveninTables5.1.4.-1and5.1.4.-2.Acceptancecriteriaarebasedonindividualresultsorontheaverageofreplicatecountswhenreplicatecountsareperformed(e.g.directplatingmethods).非无菌制剂的微生物检测按照通则2.6.12和2.6.13的方法执行。非无菌制剂的微生物限度基于表5.1.4-1和5.1.4-2的需氧微生物总数(TAMC)及酵母菌/霉菌总数(TYMC)。接受标准适用于单个检出结果或者做重复样品时结果的平均数(例如,直接平板法)。Whenanacceptancecriterionformicrobiologicalqualityisprescribeditisinterpretedasfollows:给出的微生物质量接受标准解读如下:—101CFU:maximumacceptablecount=20;—101CFU:最大可接受数=20;—102CFU:maximumacceptablecount=200;—102CFU:最大可接受数=200;—103CFU:maximumacceptablecount=2000;—103CFU:最大可接受数=2000;Table5.1.4.-1includesalistofspecifiedmicro-organismsforwhichacceptancecriteriaareset.Thelistisnotnecessarilyexhaustiveandforagivenpreparationitmaybenecessarytotestforothermicro-organismsdependingonthenatureofthestartingmaterialsandthemanufacturingprocess.表5.1.4-1给出了各种剂型的具体微生物接受标准。此表格并不完全,根据起始物料和生产工艺的性质可能需要其他的微生物检查。Ifithasbeenshownthatnoneoftheprescribedtestswillallowvalidenumerationofmicro-organismsatthelevelprescribed,avalidatedmethodwithalimitofdetectionascloseaspossibletotheindicatedacceptancecriterionisused.若所有给出的测试方法都不能有效的测出描述微生物限度,需要使用其检测限尽可能接近接受标准的经过验证的方法。Inadditiontothemicro-organismslistedinTable5.1.4.-1,thesignificanceofothermicro-organismsrecoveredisevaluatedintermsof:除了表5.1.4-1给出的微生物外,评估其他发现微生物的重要性要根据以下项目的评估:—useoftheproduct:hazardvariesaccordingtotherouteofadministration(eye,nose,respiratorytract);—natureoftheproduct:itsabilitytosupportgrowth,thepresenceofadequateantimicrobialpreservation;—methodofapplication;—intendedrecipient:riskmaydifferforneonates,infants,thedebilitated;—useofimmunosuppressiveagents,corticosteroids;—presenceofdisease,wounds,organdamage.Wherewarranted,arisk-basedassessmentoftherelevantfactorsisconductedbypersonnelwithspecialisedtraininginmicrobiologyandtheinterpretationofmicrobiologicaldata.Forrawmaterials,theassessmenttakesaccountofprocessingtowhichtheproductissubjected,thecurrenttechnologyoftestingandtheavailabilityofmaterialsofthedesiredquality.--产品使用:根据使用途径不同危害不同(眼、鼻、呼吸道);--产品的性质:促生长的能力,是否添加足量的防腐剂;--使用方法:--...