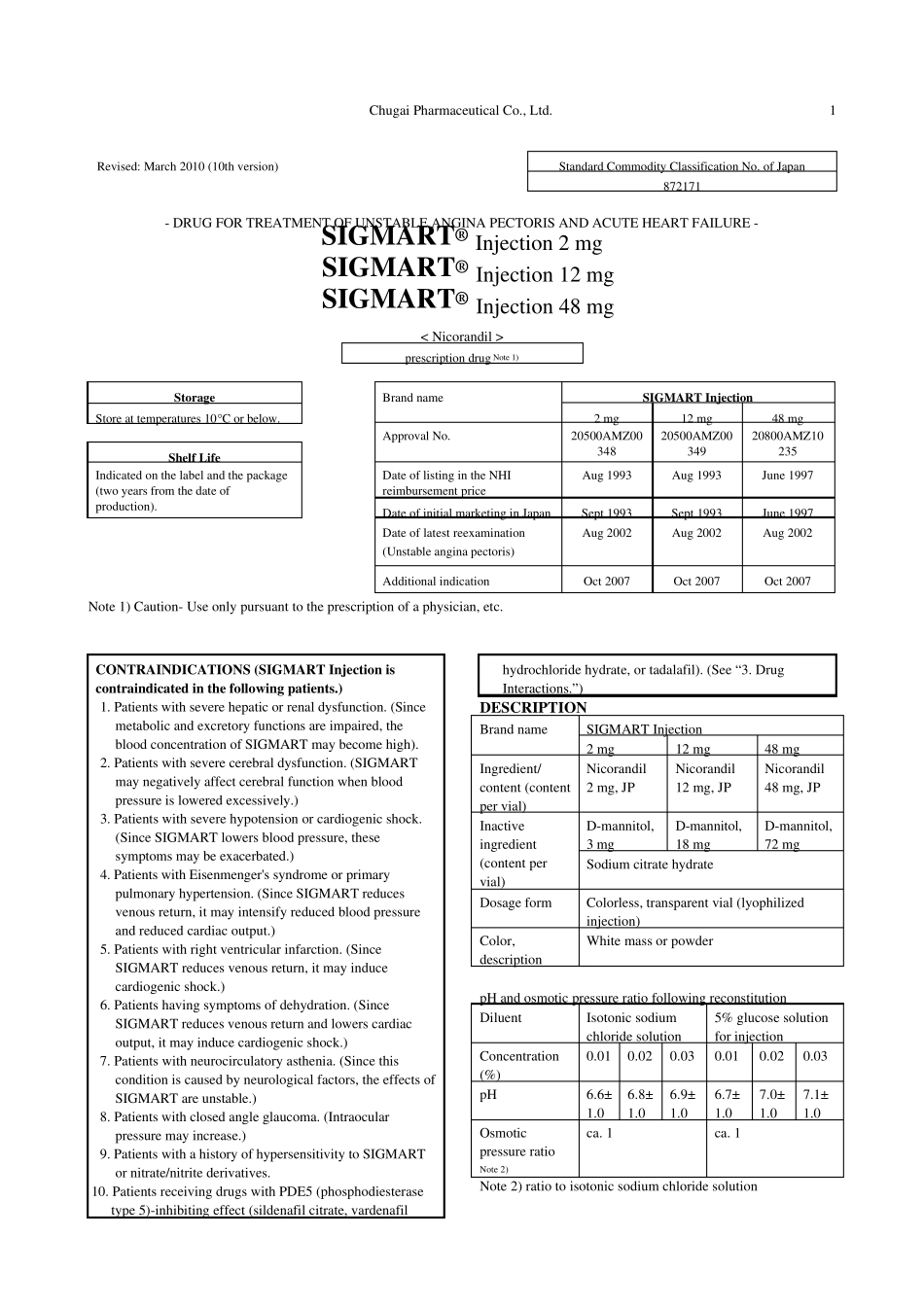
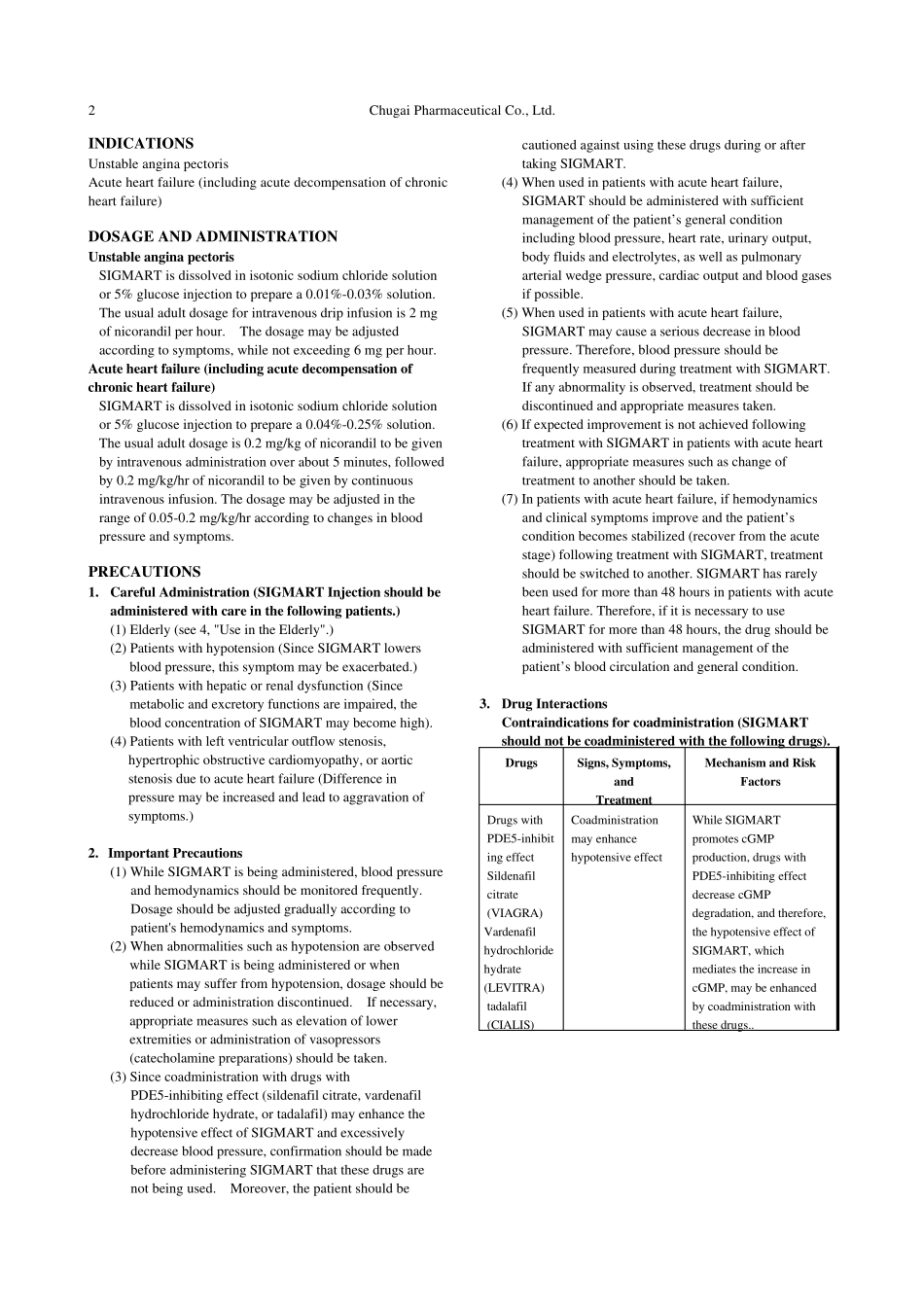
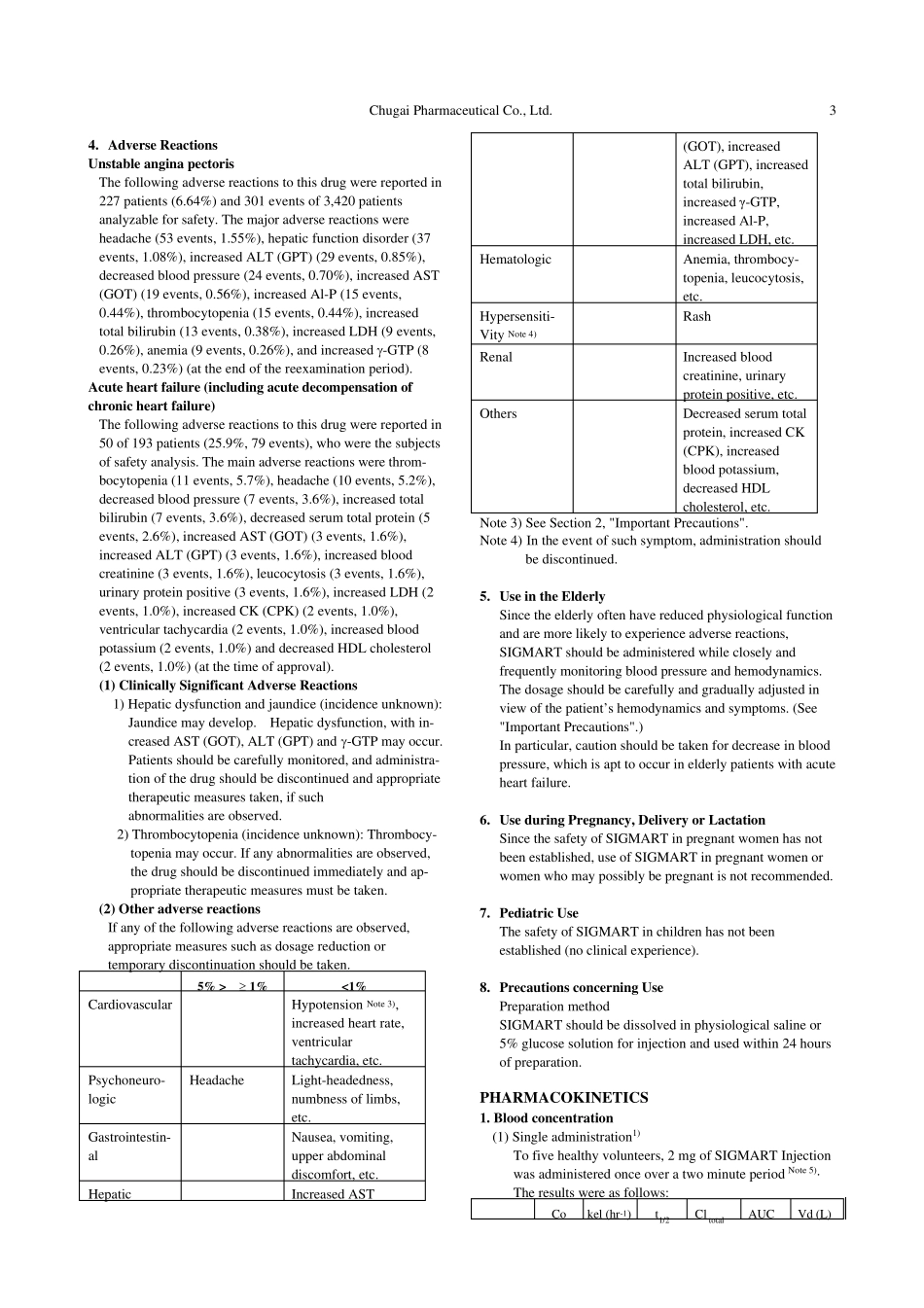
Chugai Pharmaceutical Co., Ltd. 1 Revised: March 2010 (10th version) Standard Commodity Classification No. of Japan 872171 - DRUG FOR TREATMENT OF UNSTABLE ANGINA PECTORIS AND ACUTE HEART FAILURE - SIGMART® Injection 2 mg SIGMART® Injection 12 mg SIGMART® Injection 48 mg < Nicorandil > prescription drug Note 1) Storage Brand name SIGMART Injection Store at temperatures 10°C or below. 2 mg 12 mg 48 mg Approval No. 20500AMZ00348 20500AMZ00349 20800AMZ10235 Shelf Life Indicated on the label and the package (two years from the date of production). Date of listing in the NHI reimbursement price Aug 1993 Aug 1993 June 1997 Date of initial marketing in Japan Sept 1993 Sept 1993 June 1997 Date of latest reexamination (Unstable angina pectoris) Aug 2002 Aug 2002 Aug 2002 Additional indication Oct 2007 Oct 2007 Oct 2007 Note 1) Caution- Use only pursuant to the prescription of a physician, etc. CONTRAINDICATIONS (SIGMART Injection is contraindicated in the following patients.) 1. Patients with severe hepatic or renal dysfunction. (Since metabolic and excretory functions are impaired, the blood concentration of SIGMART may become high). 2. Patients with severe cerebral dysfunction. (SIGMART may negatively affect cerebral function when blood pressure is lowered excessively.) 3. Patients with severe hypotension or cardiogenic shock. (Since SIGMART lowers blood pressure, these symptoms may be exacerbated.) 4. Patients with Eisenmenger's syndrome or primary pulmonary hypertension. (Since SIGMART reduces venous return, it may intensify reduced blood pressure and reduced cardiac output.) 5. Patients with right ventricular infarction. (Since SIGMART reduces venous return, it may induce cardiogenic shock.) 6. Patients having...