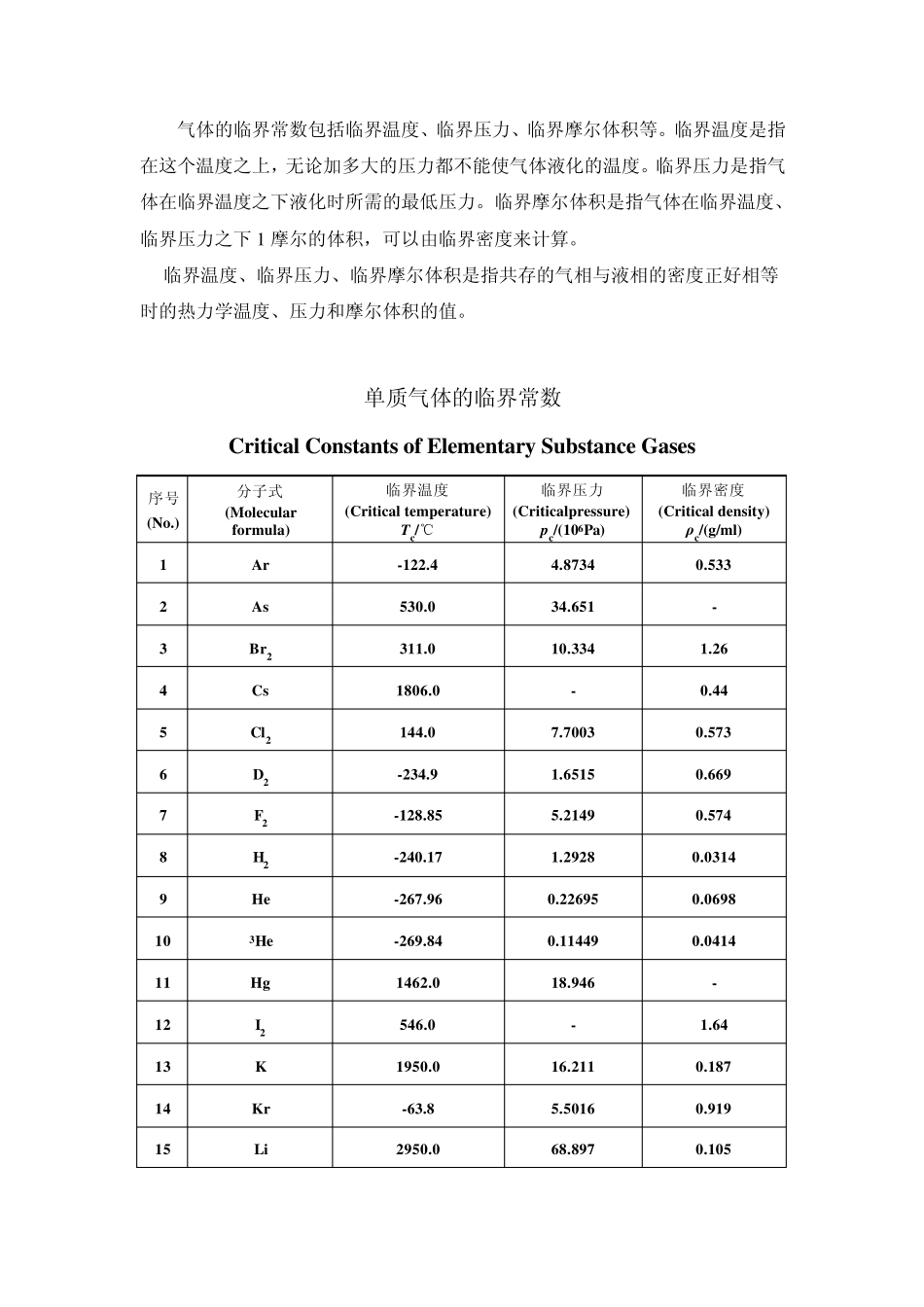
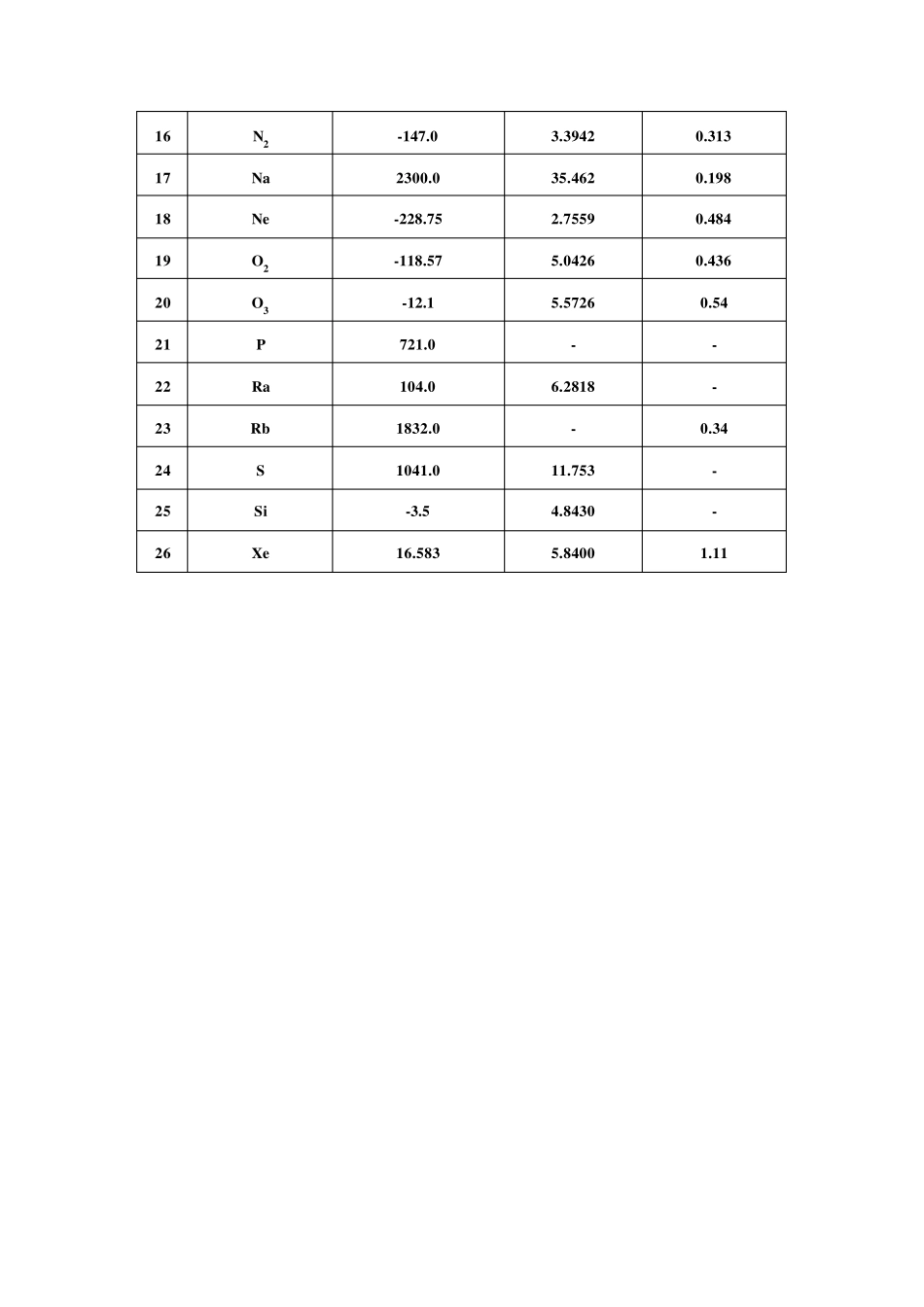
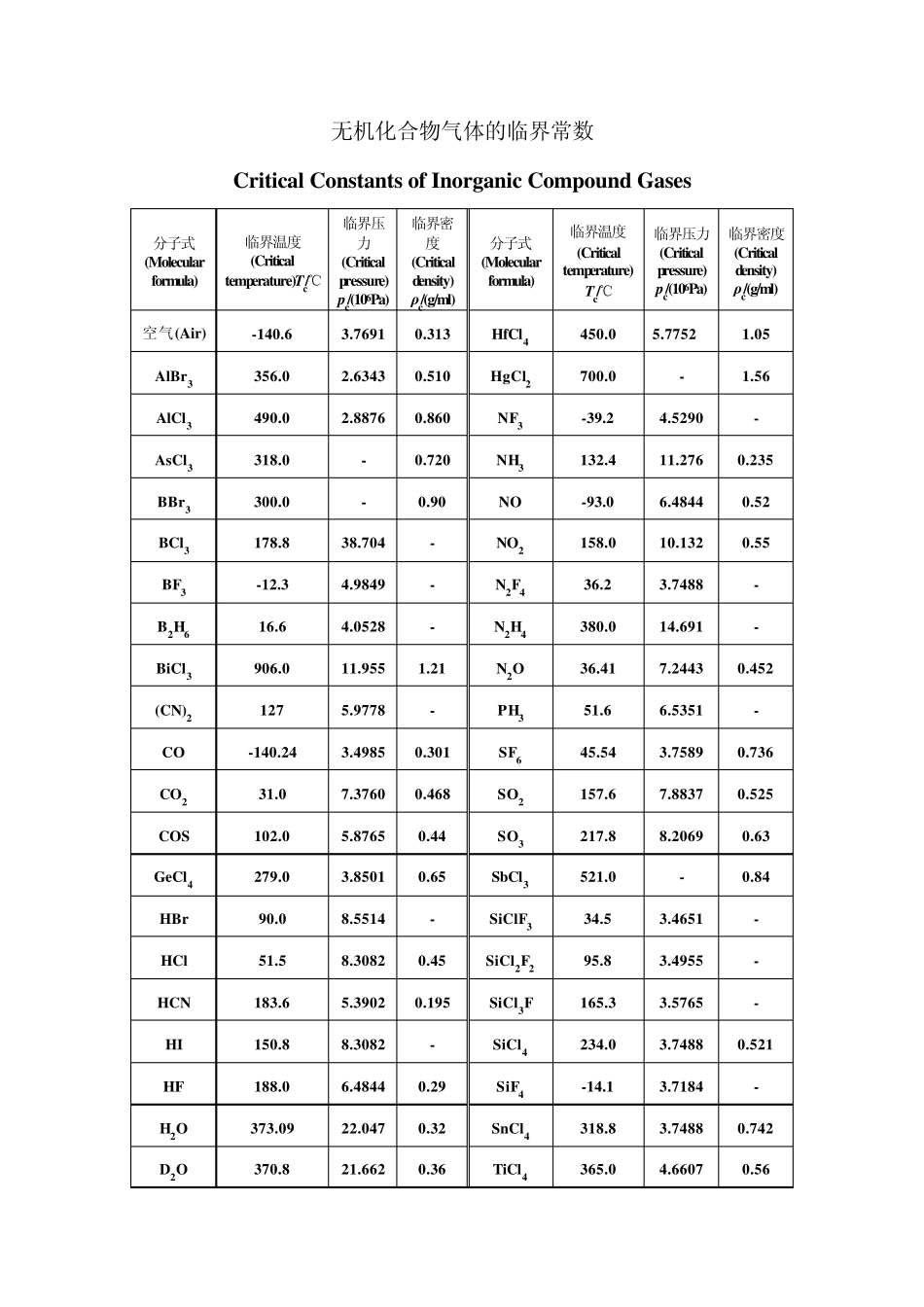
气体的临界常数包括临界温度、临界压力、临界摩尔体积等。临界温度是指在这个温度之上,无论加多大的压力都不能使气体液化的温度。临界压力是指气体在临界温度之下液化时所需的最低压力。临界摩尔体积是指气体在临界温度、临界压力之下1 摩尔的体积,可以由临界密度来计算。 临界温度、临界压力、临界摩尔体积是指共存的气相与液相的密度正好相等时的热力学温度、压力和摩尔体积的值。 单质气体的临界常数 Critical Constants of Elementary Substance Gases 序号(No.) 分子式 (Molecular formula) 临界温度 (Critical temperature) Tc/℃ 临界压力 (Criticalpressure) pc/(106Pa) 临界密度 (Critical density) ρc/(g/ml) 1 Ar -122.4 4.8734 0.533 2 As 530.0 34.651 - 3 Br2 311.0 10.334 1.26 4 Cs 1806.0 - 0.44 5 Cl2 144.0 7.7003 0.573 6 D2 -234.9 1.6515 0.669 7 F2 -128.85 5.2149 0.574 8 H2 -240.17 1.2928 0.0314 9 He -267.96 0.22695 0.0698 10 3He -269.84 0.11449 0.0414 11 Hg 1462.0 18.946 - 12 I2 546.0 - 1.64 13 K 1950.0 16.211 0.187 14 Kr -63.8 5.5016 0.919 15 Li 2950.0 68.897 0.105 16 N2 -147.0 3.3942 0.313 17 Na 2300.0 35.462 0.198 18 Ne -228.75 2.7559 0.484 19 O2 -118.57 5.0426 0.436 20 O3 -12.1 5.5726 0.54 21 P 721.0 - - 22 Ra 104.0 6.2818 - 23 Rb 1832.0 - 0.34 24 S 1041.0 11.753 - 25 Si -3.5 4.8430 - 26 Xe 16.583 5.8400 1.11 无机化合物气体的临界常数 Critical Constants of Inorganic Compound Gases 分子式(Molecular formula) 临界温度(Critical temperature)Tc/℃ 临界压力(Critical pressure) pc/(106Pa) 临界密度(Critical density) ρc/(g/ml) 分子式(Molecular formula) 临界温度 (Critical temperature) Tc/℃ 临界压力(Critical pressure) pc/(106Pa) 临界密度(Critical density) ρc/(g/ml) 空气(Air) -140.6 3.7691 0.313 HfCl4 450.0 5.7752 1.05 AlBr3 356.0 2.6343 0.510 HgCl2 700.0 - 1.56 AlCl3 490.0 2.8876 0.860 NF3 -39.2 4.5290 - AsCl3 318.0 - 0.720 NH3 132.4 11.276 0.235 BBr3 300.0 - 0.90 NO -93.0 6.4844 0.52 ...