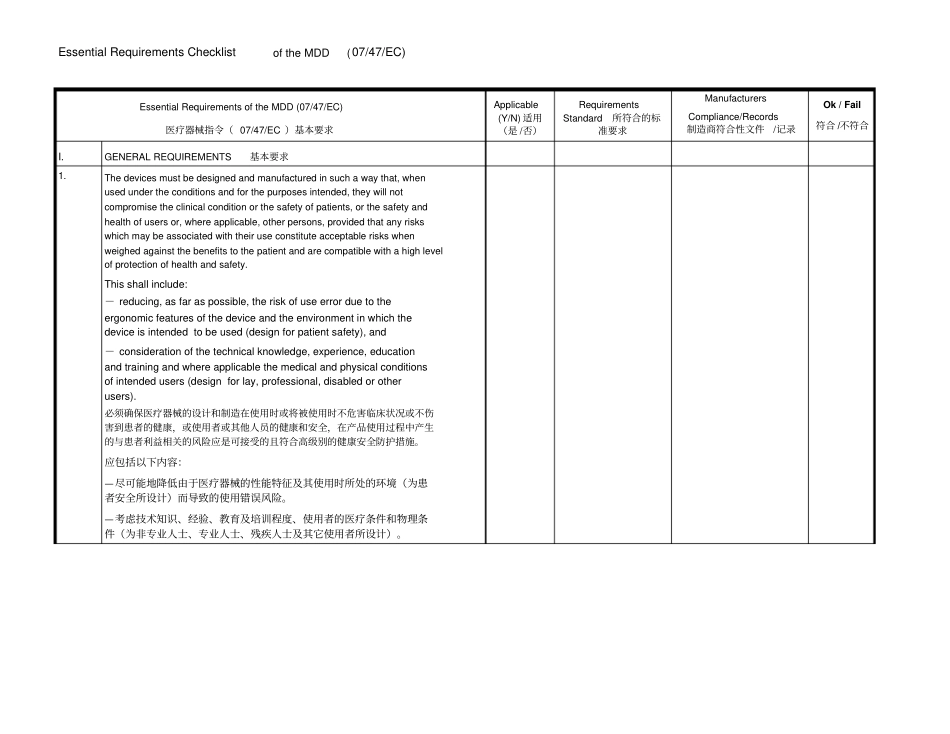
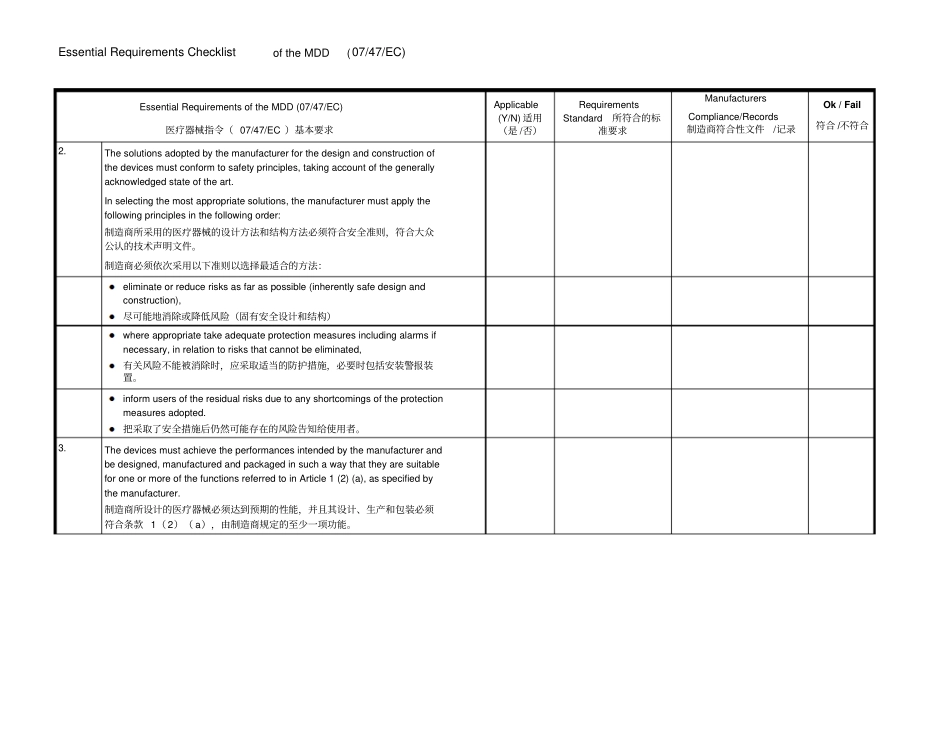
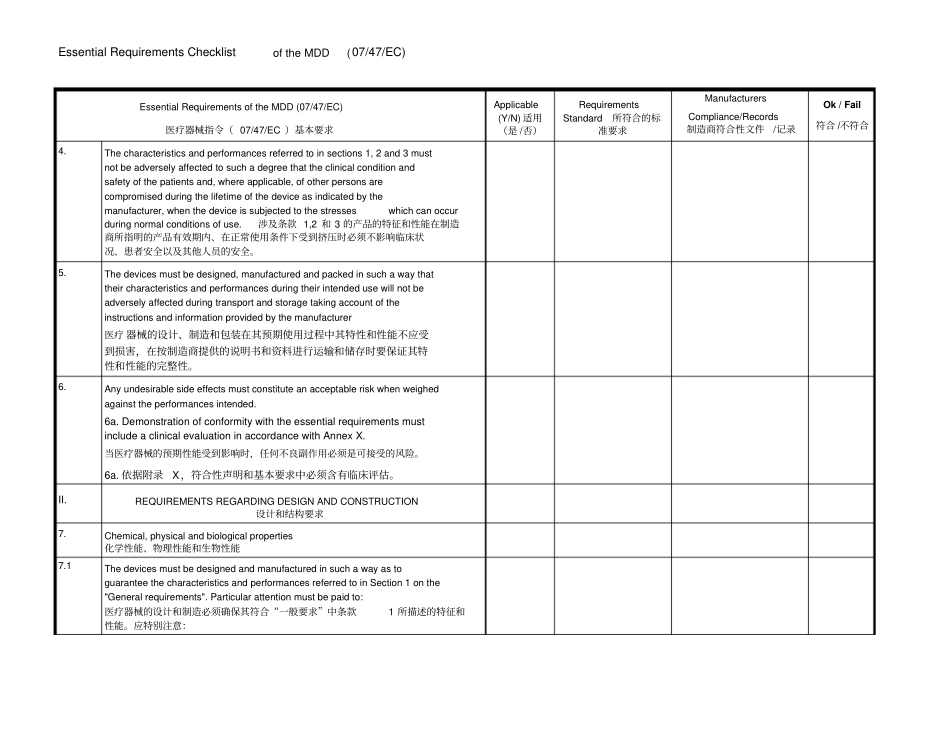
Essential Requirements Checklist of the MDD (07/47/EC) Essential Requirements of the MDD (07/47/EC) 医疗器械指令( 07/47/EC )基本要求Applicable (Y/N) 适用(是 /否)Requirements Standard 所符合的标准要求Manufacturers Compliance/Records 制造商符合性文件/记录Ok / Fail 符合 /不符合I. GENERAL REQUIREMENTS基本要求1.The devices must be designed and manufactured in such a way that, when used under the conditions and for the purposes intended, they will not compromise the clinical condition or the safety of patients, or the safety and health of users or, where applicable, other persons, provided that any risks which may be associated with their use constitute acceptable risks when weighed against the benefits to the patient and are compatible with a high level of protection of health and safety.This shall include:- reducing, as far as possible, the risk of use error due to the ergonomic features of the device and the environment in which the device is intended to be used (design for patient safety), and - consideration of the technical knowledge, experience, education and training and where applicable the medical and physical conditions of intended users (design for lay, professional, disabled or other users). 必须确保医疗器械的设计和制造在使用时或将被使用时不危害临床状况或不伤害到患者的健康,或使用者或其他人员的健康和安全,在产品使用过程中产生的与患者利益相关的风险应是可接受的且符合高级别的健康安全防护措施。应包括以下内容:—尽可能地降低由于医疗器械的性能特征及其使用时所处的环境(为患者安全所设计)而导致的使用错误风险。—考虑技术知识、经验、教育及培训程度、使用者的医疗条件和物理条件(为非专业人士、专业人士、残疾人士及其它使用者所设计)。Essential Requirements Checklist of the MDD (07/47/EC) Essential Requirements of the MDD (07/47/EC) 医疗器械指令( 07/47/EC )基本要求Applicable (...