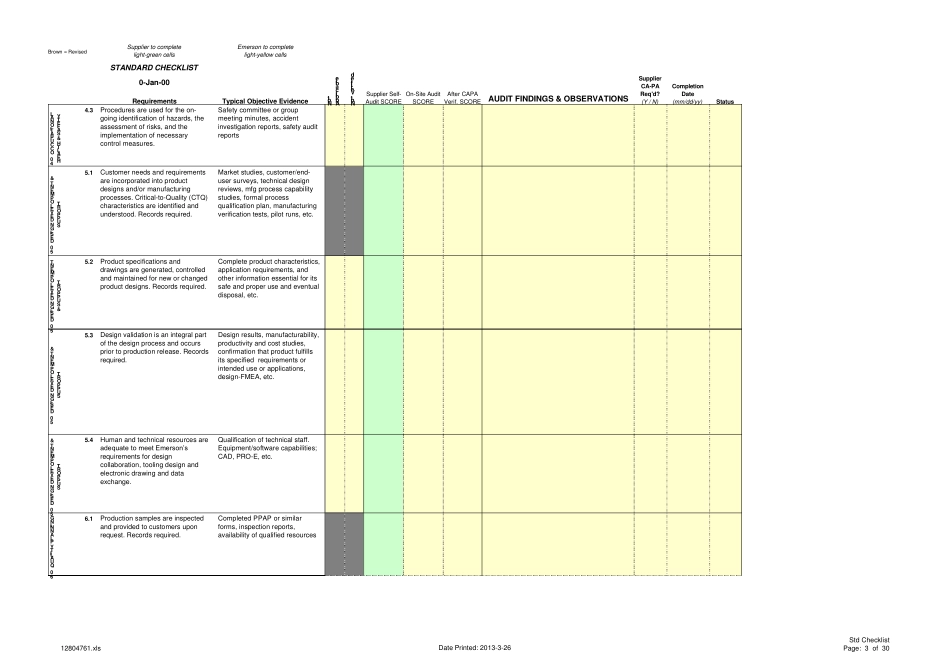
Supplier to completelight-green cellsEmerson to completelight-yellow cellsSTANDARD CHECKLIST0-Jan-00RequirementsTypical Objective EvidenceAUDIT FINDINGS & OBSERVATIONSNotApplicableNot VerifiedSupplier Self-Audit SCORESupplierCA-PAReq'd?(Y / N)On-Site AuditSCOREAfter CAPAVerif. SCORECompletionDate(mm/dd/yy)StatusBrown = Revised1.0 QUALITY MANAGEMENT1.1The quality system is documented,controlled, and maintained toclearly describe current practice.Documented procedures required.Records required.Quality manual and all QSprocedures show revision control(sign-offs & dates), history ofchanges, quality organization'sresponsibilities1.0 QUALITY MANAGEMENT1.2Quality reports, trend charts anddata analysis identify areas ofopportunity and are used bymanagement on a routine basis.Records required.Product quality yield data, topproblems and correspondingimprovement actions, status ofpreventive/corrective actionstaken, internal audit results1.0 QUALITYMANAGEMENT1.3Quality performance targets areclearly defined, included in thebusiness plan and monitored forimprovements.Strategic and tactical objectives,goals, action plans, etc.1.0 QUALITY MANAGEMENT1.4Executive management participatesin periodic quality system reviewsthat address quality relatedfeedback from customers andinternal quality metrics. Recordsrequired.Analysis of field failures,inspection yields, resourceneeds, internal audit results,corrective action status, etc.2.0 CONTINUOUS IMPROVEMENT2.1Preventive actions are taken basedon the analysis of significantbusiness trends, design reviews,customer satisfaction surveys orother meaningful inputs.Documented procedures required.Records required.Management review meetings,goal setting, performancemeasurement, internal audits,action plans, customer surveys2.0 CONTINUOUS IMPROVEMENT2.2A formal approach is used toac...