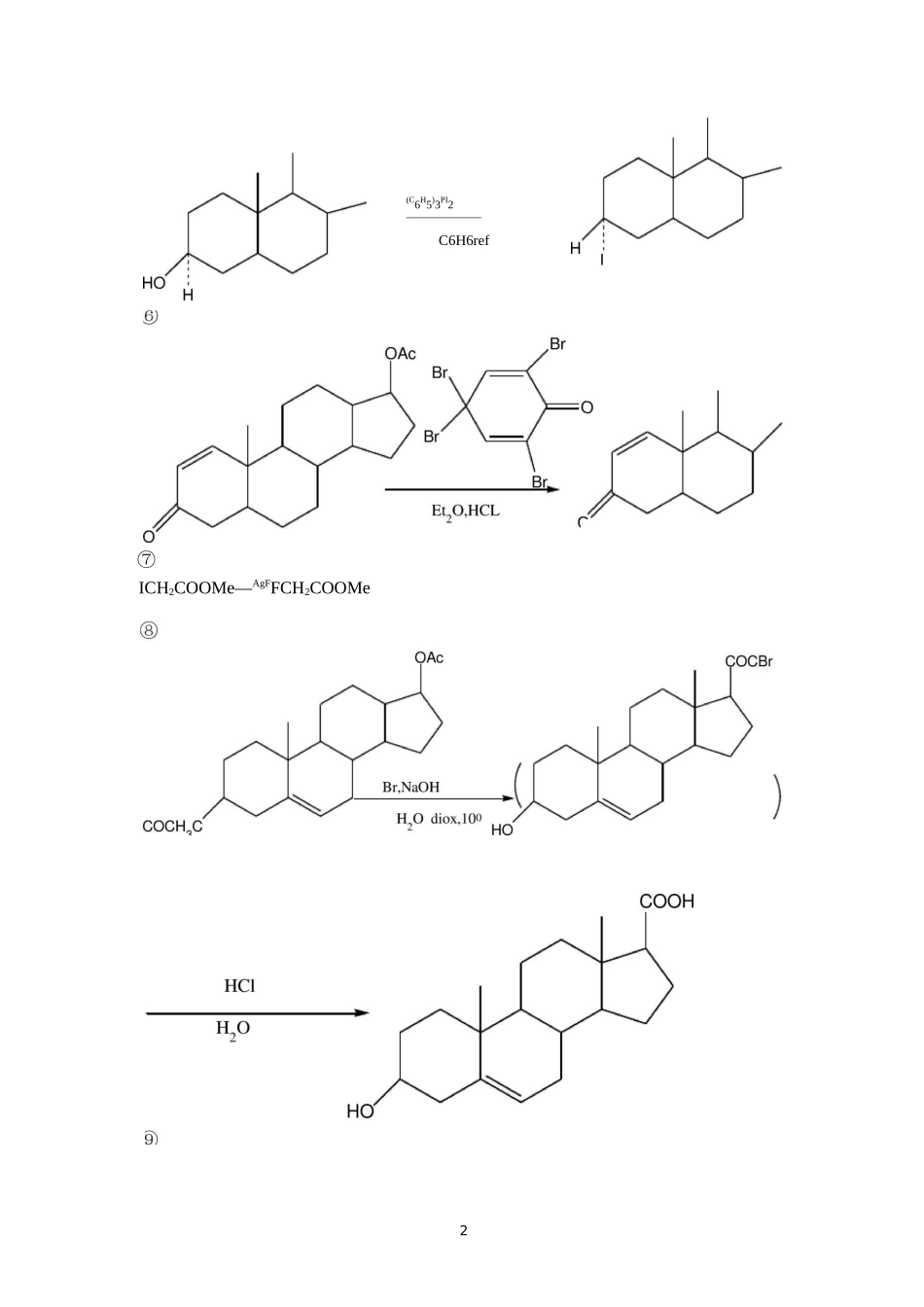
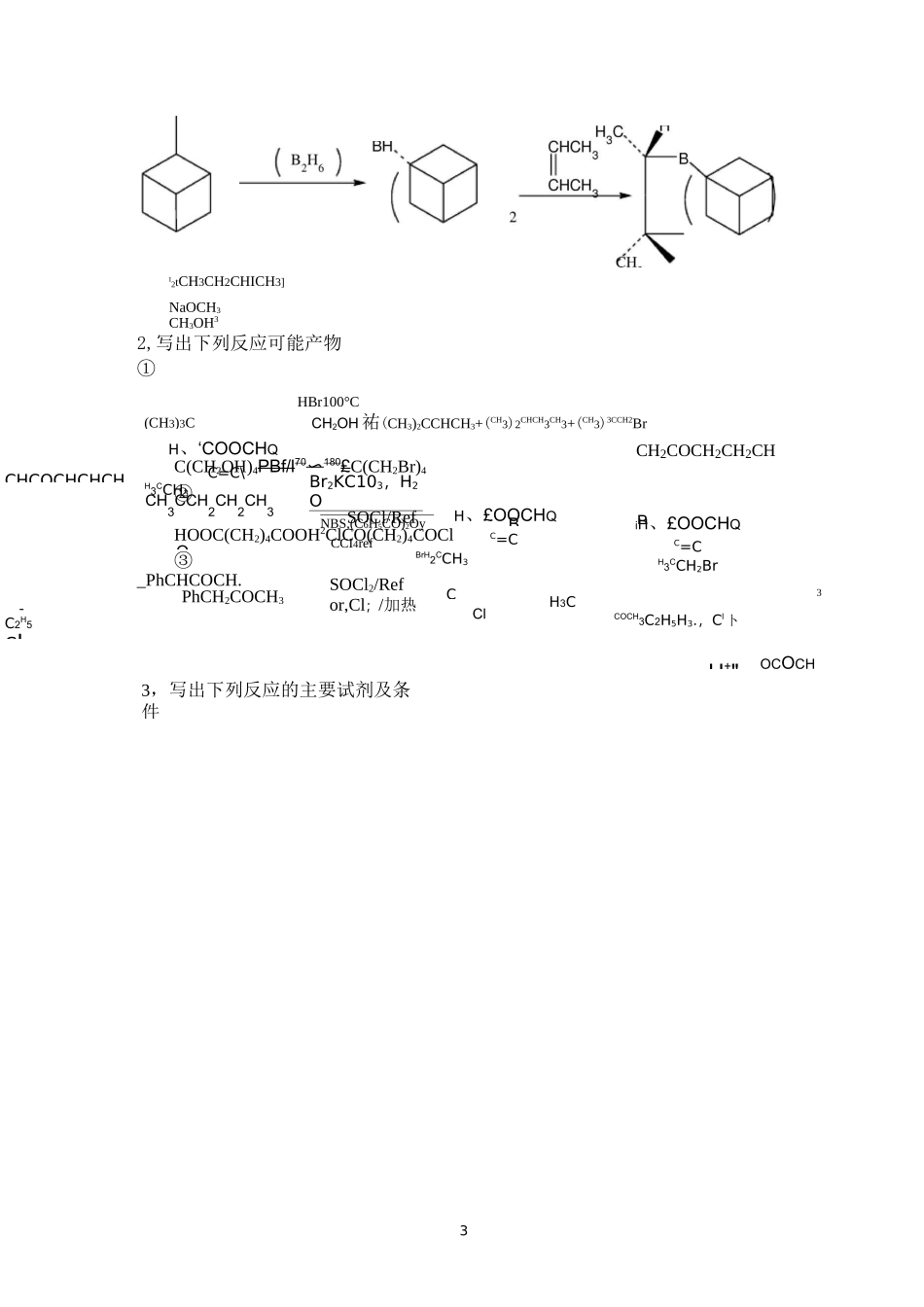
1第一章卤化反应1,写出下列反应的主要产物(或试剂)3Me2(C6H5)3PI2C6H6refI⑦ICH2COOMe―AgFFCH2COOMe(CH3)3CH、‘COOCHQC=C\H3CCH3NBS,(C6H5CO)2OyCCI4refH、£OOCHQC=CBrH2CCH3iH、£OOCHQC=CH3CCH2BrCH3CCH2CH2CH3OBr2KC103,H2O40〜45CCHCOCHCHCHBCH2COCH2CH2CHBCH3C、COCH3C2H5H3.,Cl卜H+"-C2H5CL3,写出下列反应的主要试剂及条件OCOCH3PhCH2COCH3SOCl2/Refor,Cl;/加热3I2tCH3CH2CHICH3]NaOCH3CH3OH32,写出下列反应可能产物①HBr100°CCH2OH祐(CH3)2CCHCH3+(CH3)2CHCH3CH3+(CH3)3CCH2BrC(CH2OH)4PBf/l70〜180£C(CH2Br)4②SOCl/RefHOOC(CH2)4COOH2ClCO(CH2)4COCl③_PhCHCOCH.3Cl4第二章烃化反应4,完成下列反应①OHCH3INaOHOHOHMe2SO4K2CO3OHHH3COCHOCH32——CH2OHH2H3C——C2—CH2OHOH5(H2C)5NHMe2SO425--HcNC2HC^MO⑤O2NClNO2PhNH22―■95°CNHPhCH2OHNHCH262,完成下列反应过程①O2CH2COOC2H5HClH£C2H5ONaC2H5OHN—C(COOC2H5)2NaCH2COOHCHCOOHH2NCH2NHCH3C(COOC2H5)2CHCOOHC6H5CH2NH2(呼询.C6H5CH2NH0(OC2H5)2H2CCHCH2Br/50%NaOH.(n-C4Hg)4NHSO4④护C6H5CH2NP(OC2H5)2CH2CH二CH2HClC6H5CH2N652.HClCH2CH=CH2O2N③7HNHCOCHCI2C——C——CH2OH+CH3(CH2)16COClOHHHNHCOCHCI2CCCH2OOC(CH2)16CH3OHHO2N(H3C)3CCH3CH3第三章酰化反应3CH3COClCH338(CH2)5COClZn(CN)2/HCl①DMF/POC1?-②H2O⑥CH3+C6H5N一CHOP0C3A6590〜95°C92,完成下列合成路线CH3COCH2COOC2H5140°C4〜5小时②以苯和丁二酸为起始原料合成四氢萘AlCl3Zn-HgHC110第四章缩合COCH3+HCHO+CH3NH3CH3COCH2CH2N-2H3Ph3PCHCH碱CH3CH2CH2COCH3+CH3COOC2H5④aCH3CH2CH2COCH2COCH3①Zn+C6H6CHO+BrCH2COOC2H5(C2H5)2O②H2oC^CHCOOCH252,完成下列反应式①CH3+O2NcHONO211OO2Ph严CHCH3H3CHCCHCH3O+CH2O(过量)-|O竺CHCHO①(C2H5CH2CO)2O,C3H7COOK65②NaOH3OH2O-OH(EtO)2C=O+C6H5CH2CNC?H5ON^CH65HC——C——OCH25CN(C6H5)3NaNH—PCKCH=CH2Cl22液NH3C6H6回流©(C6H5)3—PO-CHCH=CH2123,完成下列合成反应①从甲苯fH3C6H625%66H33C6H6(C2H5)2OZnCH3CO2HH2OCH3COOEt」COOEt2H5NaCNBr/hvEtONaC2H5BrCOOEtCOOEt/COOEtCHOOC2H5CH2BrCOOC-HEtOHH-CH2CNCOOEtCNO加热/COOEtC2H5COOEtCOOC2H5H+13CH33CHOCCCHOCH3HCH34,填写下列各部产物①HCHO/OH口一甲基丙醛HCNA(C5H10O2^^^B(C6H12O4)+HQ一—*C(C6H10O3)NHCHCHCOOCHPhD三HO+3E(C9H17NO5)+PhCH2OHAH3C严0日CCHOCH3CH2OH/OHH3CC—CHCH3CNH3C—OH2C—OCH2OHOHH3C——CCCH3OHNCH2CH2COOCH2PhCH2OHHH3C——C—CNCH2CH2COOHCH3OH143第五章氧化反应完成下列反应式1,②CrO3/Ac2OH2SO415④⑤NO2CrO2Cl2CS225“CNO2(11)160.5MSeO2C2H5°H回流⑩RCO3HO(C172,试以化学表示实现下列变化的各步反应①DMSOOHC(CH2)5CH3③CHO稀NaOHC2H5CH°Ag2OCOOC2H5C2H5CONH2C2H5CONSO2ClH3SO2O(CH2)6CH3H3COOOH(C2H5)2O25°CQHH3C'H3CH3318第六章还原反应1,完成下列反应①OH④OHPb(OAc)4OH©H2SO②KMnOOCHOOH①CrO3H2SO4H2Q②SeO;4Bu-tO①KQH②HCl,加热Bu-tCHOCHOOBu-t0.25MNaBHCH3CH=CHCHQ4”CH3CH=CHCH2QH2Br19HH2O2NaOH厂Pb-BaSO4H242・Q-S⑦2PHCH2CH=CH2+Pd/C2PHCH2CH2CH3+H2/Pd-CAcOHHCQHOCH2CH2OCH2CH2PhLA202,改错①CH3COCOOEtZn-HgHC1严CH3CHOHCOOEt③HJIHH3CHH3CCH3CH33,完成下列合成题HC——C=O2|I1,试由环己醇、氯乙酸、乙醇合成(屯宓一CH?CH3LAHHOOCCOOEtLAHHOH2CCOOEtHOH2CCH2OHCl(CH2)6Cl②EtOHH+21CH2(COOC2H5)22CH2(COOC2H5)2-Cl(CH2)6ClJHfNa.^电0。—①H2ONaOHCH(CH2)6CH(COOC2H5)2-①7-C2H5OOC(CH2)8COOC2H5OHClCH2COOH2严4化)2・=Oref2Zn/HClH2C——C=OHOAc(H2C)7——CH2O2,试由合成OOCH3,试由制备H(H2C)7——CHOHCH2CH2COOHOcH30.25MNaBHHCOOEtO221,完成下列反应式COOEtNaC2H5OHCHCHCOOHOHCH2CH2COONaHCH2OHH+第七章重排反应cCONHCONH233④-C广PhLi/PhH加热CH3CH2N(CH3)3NaNH2/NH3CH2N(CH3)2CH3OH⑦H+-H2°3OCH3NaOCl/NaOHOCH324NC180°CEtOOC2NaNO2/H+H3(ID25CH3C(OEt)3142〜147°C8days(12)2二,完成下列合成过程O265加热H加热O332733NaBH4CH3OH