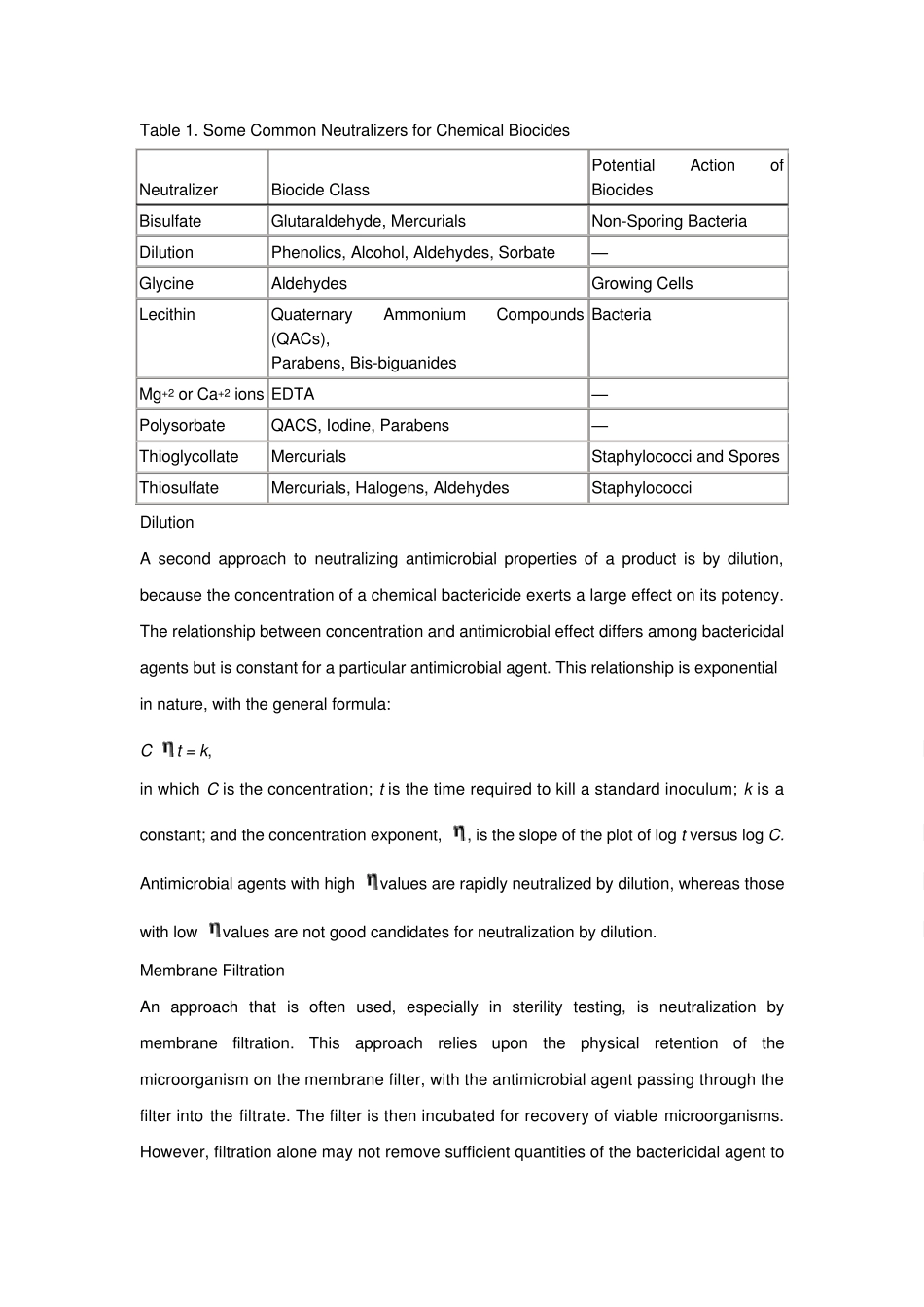
1227 VALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES This chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for the detection of indicators or objectionable microorganisms, for the validation of microbiological methods used in antimicrobial effectiveness testing, and for the sterility testing of Pharmacopeial articles. It is generally understood that if a product possesses antimicrobial properties because of the presence of a specific preservative or because of its formulation, this antimicrobial property must be neutralized to recover viable microorganisms. This neutralization may be achieved by the use of a specific neutralizer, by dilution, by a combination of washing and dilution, or by any combination of these methods. The tests under Antimicrobial Effectiv eness Testing 51, Sterility Tests 71, and Microbial Limit Tests 61 require the validation of recovery methods. To ensure that the results of the tests are credible, neutralization of antimicrobial properties of the test solution is required before estimating the number of viable microorganisms. INFLUENTIAL FACTORS Several factors affect the measurement of a test solution's antimicrobial activity, and these must be considered in the validation design. They include the nature of the microorganisms used as challenge organisms, the preparation of the inoculum of challenge organisms, the specific conditions of the test, and the conditions of recovery. These factors also affect the validation of recovery methods for aqueous or nonaqueous products, irrespective of their antimicrobial properties; thus, all test methods should be validated with these factors in mind. The nature of the chal...