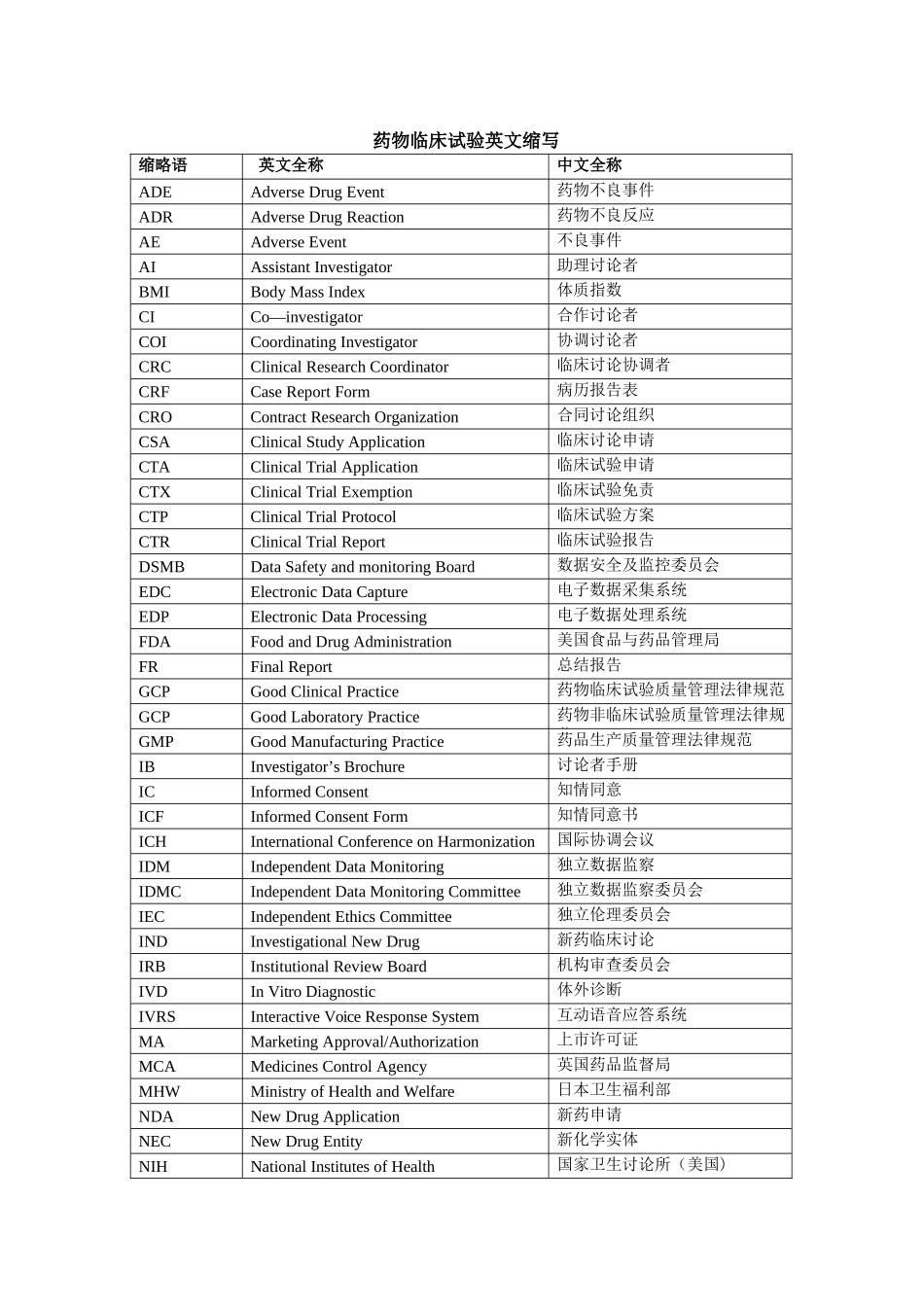
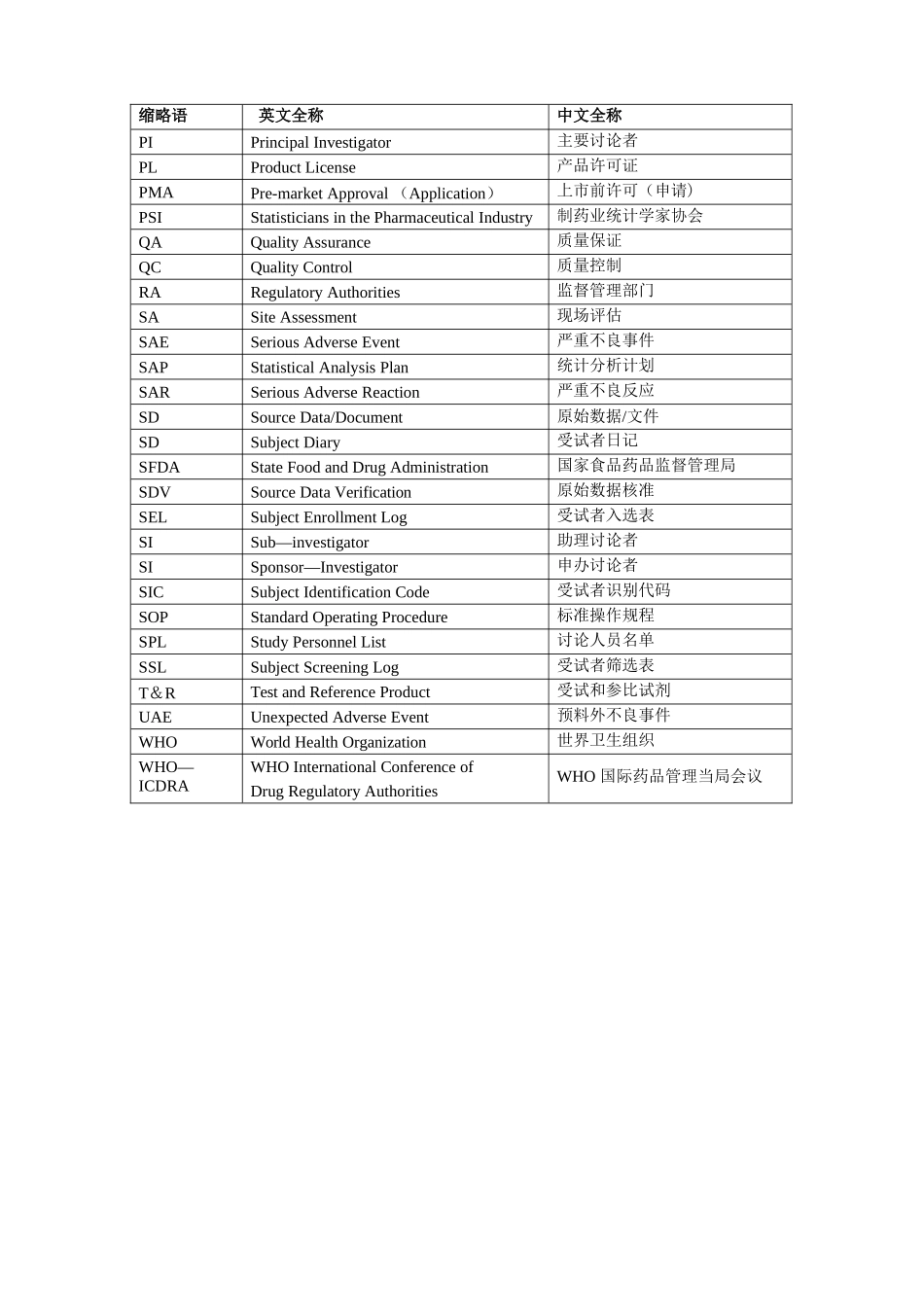
中国创新药咨询与服务先锋 CRO临床试验以及实验室中常见的英文缩写药物临床试验英文缩写缩略语英文全称中文全称ADEAdverse Drug Event药物不良事件ADRAdverse Drug Reaction药物不良反应AEAdverse Event不良事件AIAssistant Investigator助理讨论者BMIBody Mass Index体质指数CICo—investigator合作讨论者COICoordinating Investigator协调讨论者CRCClinical Research Coordinator临床讨论协调者CRFCase Report Form病历报告表CROContract Research Organization合同讨论组织CSAClinical Study Application临床讨论申请CTAClinical Trial Application临床试验申请CTXClinical Trial Exemption临床试验免责CTPClinical Trial Protocol临床试验方案CTRClinical Trial Report临床试验报告DSMBData Safety and monitoring Board数据安全及监控委员会EDCElectronic Data Capture电子数据采集系统EDPElectronic Data Processing电子数据处理系统FDAFood and Drug Administration美国食品与药品管理局FRFinal Report总结报告GCPGood Clinical Practice药物临床试验质量管理法律规范GCPGood Laboratory Practice药物非临床试验质量管理法律规范GMPGood Manufacturing Practice药品生产质量管理法律规范IBInvestigator’s Brochure讨论者手册ICInformed Consent知情同意ICFInformed Consent Form知情同意书ICHInternational Conference on Harmonization国际协调会议IDMIndependent Data Monitoring独立数据监察IDMCIndependent Data Monitoring Committee独立数据监察委员会IECIndependent Ethics Committee独立伦理委员会INDInvestigational New Drug新药临床讨论IRBInstitutional Review Board机构审查委员会IVDIn Vitro Diagnostic体外诊断IVRSInteractive Voice Response System互动语音应答系统MAMarketing Approval/Authorization上市许可证MCAMedicines Control Agency英国药品监督局MHWMinistry of Health and Welfare日本卫生福利部NDANew Drug Application新药申请NECNew Drug Entity新化学实体NIHNational Institutes of Health国家卫生讨论所(美国)缩略语英文全称中文全称PIPrincipal Investigator主要讨论者PLProduct License产品许可证PMAPre-market Approval (Application)上市前许可(申请)PSIStatisticians in the Pharmaceutical Industr...