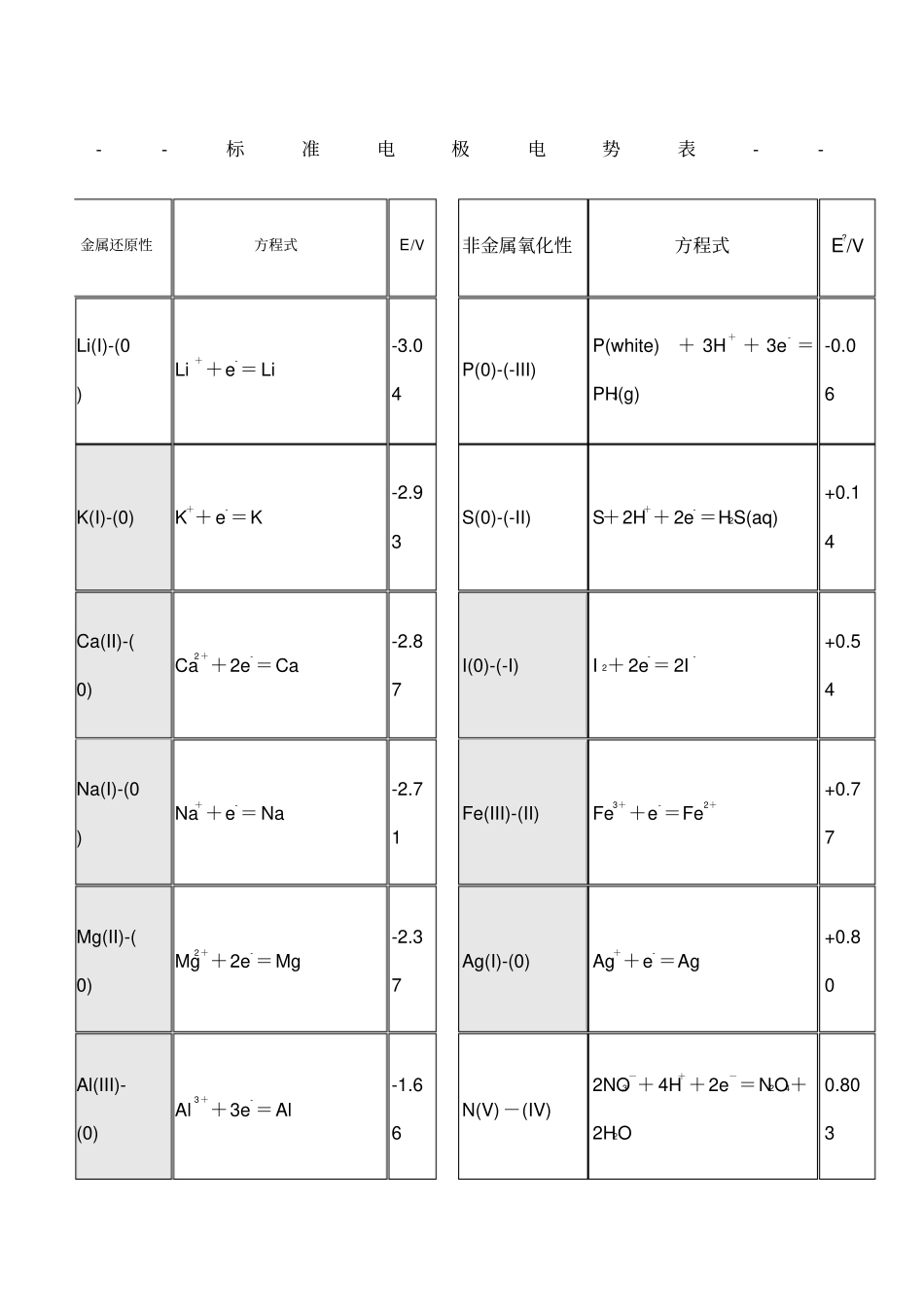
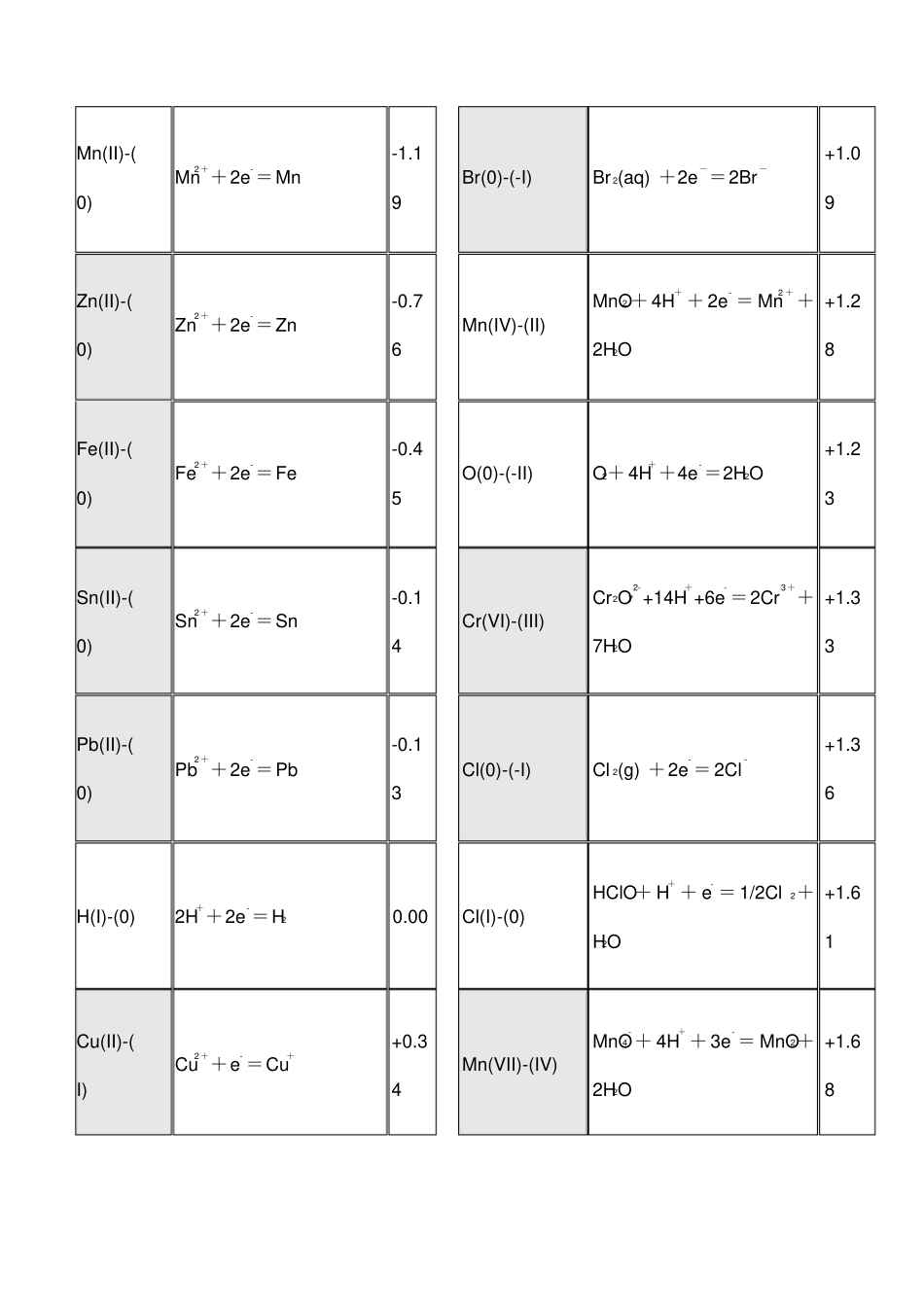
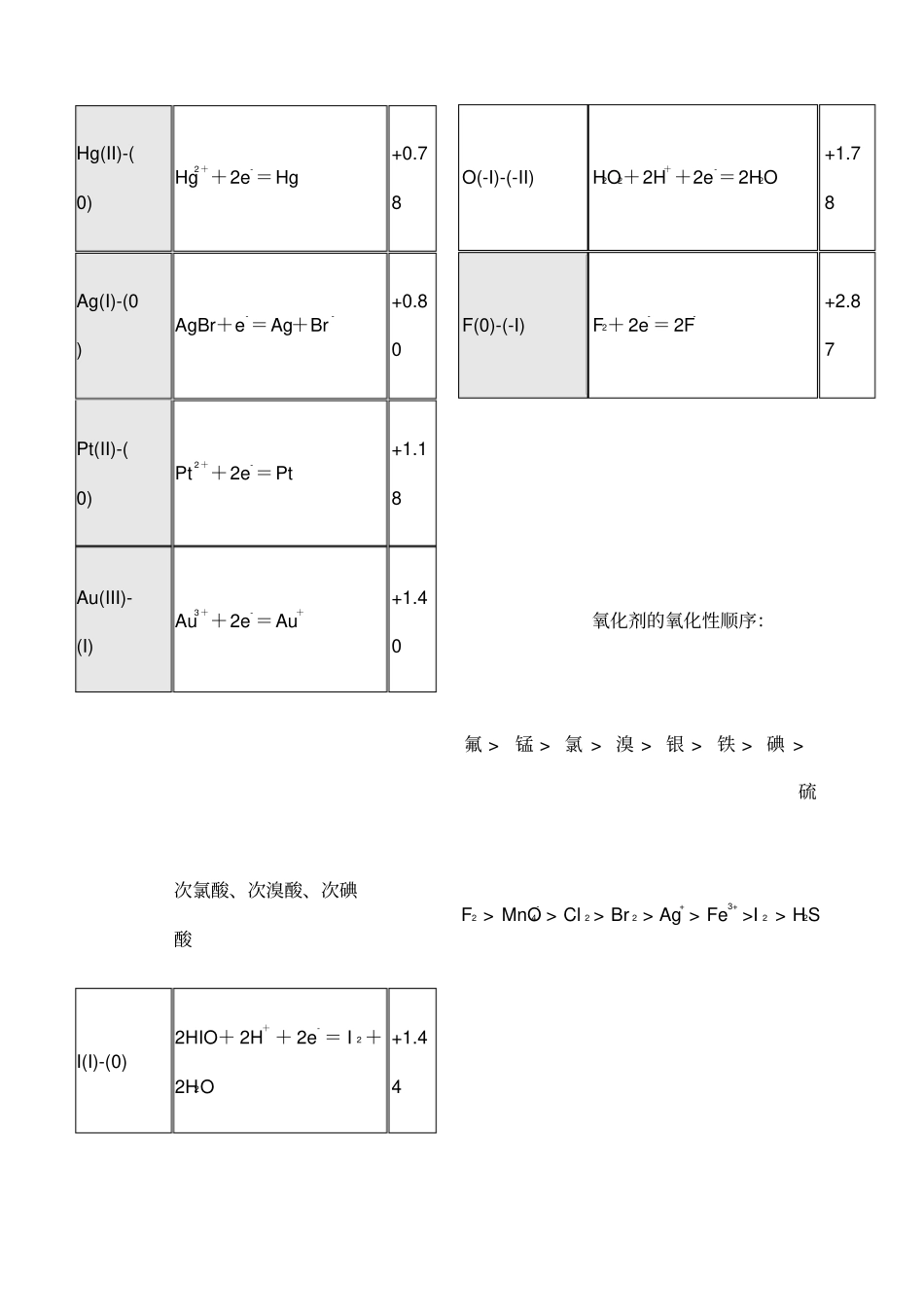
--标准电极电势表--金属还原性方程式E/V 非金属氧化性方程式E?/VLi(I)-(0)Li++e-= Li-3.04P(0)-(-III)P(white)+ 3H+ + 3e- =PH3(g)-0.06K(I)-(0)K++ e-=K-2.93S(0)-(-II)S+2H++ 2e- =H2S(aq)+0.14Ca(II)-(0)Ca2++2e-=Ca-2.87I(0)-(-I)I 2+ 2e-= 2I-+0.54Na(I)-(0)Na++e-= Na-2.71Fe(III)-(II)Fe3++e-=Fe2++0.77Mg(II)-(0)Mg2++2e-=Mg-2.37Ag(I)-(0)Ag++e- =Ag+0.80Al(III)-(0)Al3++3e-=Al -1.66N(V) -(IV) 2NO3-+ 4H++2e-=N2O4+2H2O 0.803Mn(II)-(0)Mn2++2e-=Mn-1.19Br(0)-(-I)Br 2(aq) +2e-=2Br-+1.09Zn(II)-(0)Zn2++2e-=Zn-0.76Mn(IV)-(II)MnO2 + 4H+ + 2e- = Mn2 + +2H2O+1.28Fe(II)-(0)Fe2++2e-=Fe-0.45O(0)-(-II)O2+ 4H++4e-=2H2O+1.23Sn(II)-(0)Sn2++2e-=Sn-0.14Cr(VI)-(III)Cr2O72-+14H++6e- =2Cr3++7H2O+1.33Pb(II)-(0)Pb2++2e-=Pb-0.13Cl(0)-(-I)Cl 2(g) +2e-= 2Cl-+1.36H(I)-(0)2H++2e-=H20.00Cl(I)-(0)HClO+ H+ + e- = 1/2Cl2+H2O+1.61Cu(II)-(I)Cu2++e- =Cu++0.34Mn(VII)-(IV)MnO4- + 4H+ + 3e- = MnO2+2H2O+1.68Hg(II)-(0)Hg2++2e-=Hg+0.78O(-I)-(-II)H2O2+2H++2e-=2H2O+1.78Ag(I)-(0)AgBr+e-=Ag+Br-+0.80F(0)-(-I)F2+ 2e-= 2F-+2.87Pt(II)-(0)Pt2++2e-=Pt+1.18Au(III)-(I)Au3++2e-=Au++1.40氧化剂的氧化性顺序:氟 > 锰 > 氯 > 溴 > 银 > 铁 > 碘 > 硫次氯酸、次溴酸、次碘酸F2 > MnO4- > Cl 2 > Br 2 > Ag+ > Fe3+ >I 2 > H2SI(I)-(0)2HIO+ 2H+ + 2e- = I 2 +2H2O+1.44Br(I)-(0)HBrO+H++e-=l/2Br2(aq) +H2O+1.57Cl(I)-(0)HClO+ H+ + e- = 1/2Cl2+H2O+1.61亚氯酸、高氯酸Cl(VII)-(0)ClO4- + 8H+ +7e- = 1/2Cl2+4H2O+1.39Cl(III)-(-I)HClO2+3H++4e- =Cl- +2H2O+1.57溴酸、氯酸、碘酸I(V)-(0)2IO3- + 12H+ + 10e- = I 2+6H2O+1.20Cl(V)-(-ClO3- +6H++ 6e- =Cl- + +1.4I)3H2O5Br(V)-(0)BrO3- + 6H+ +5e- = l/2Br2+3H2O+1.48