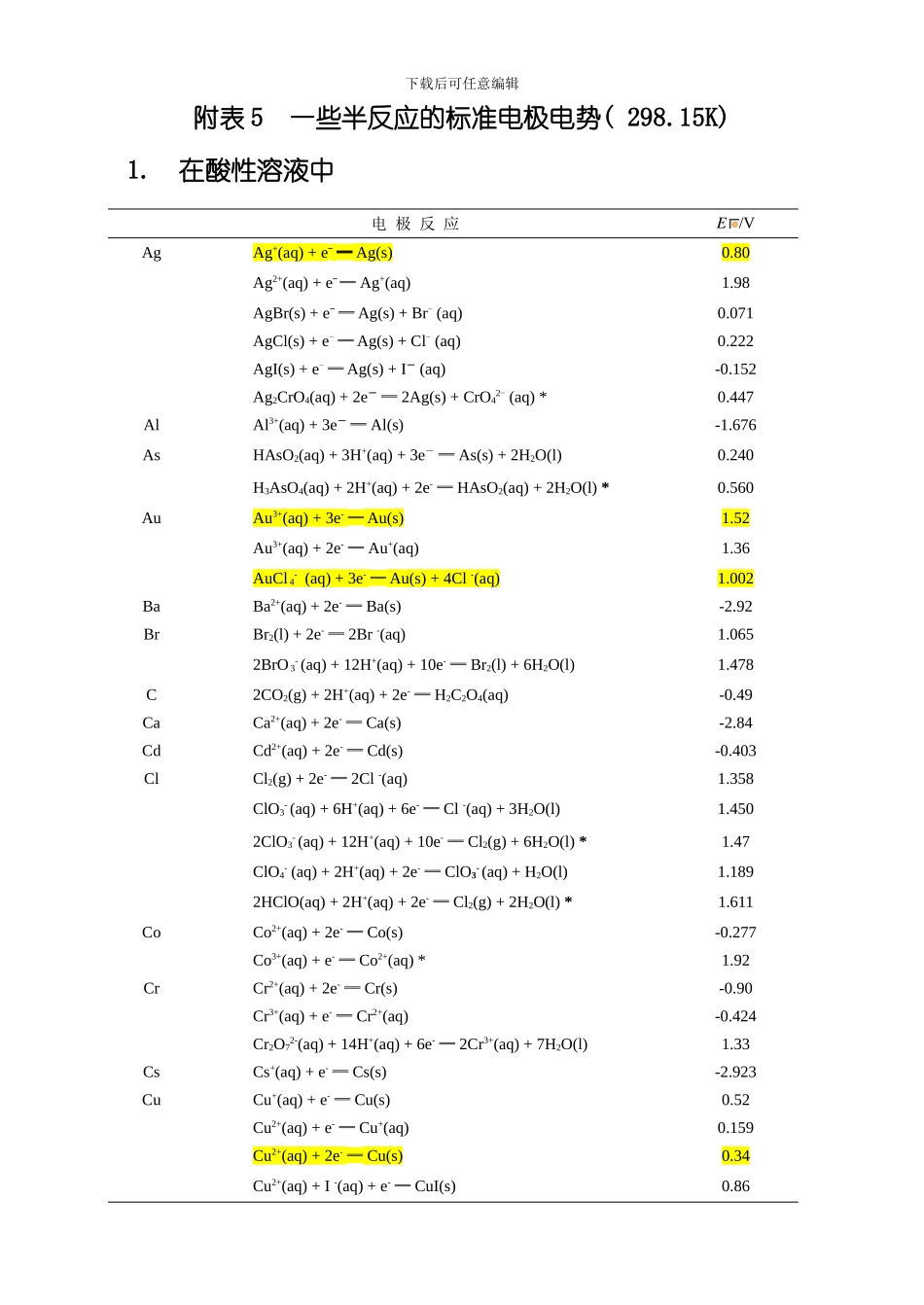
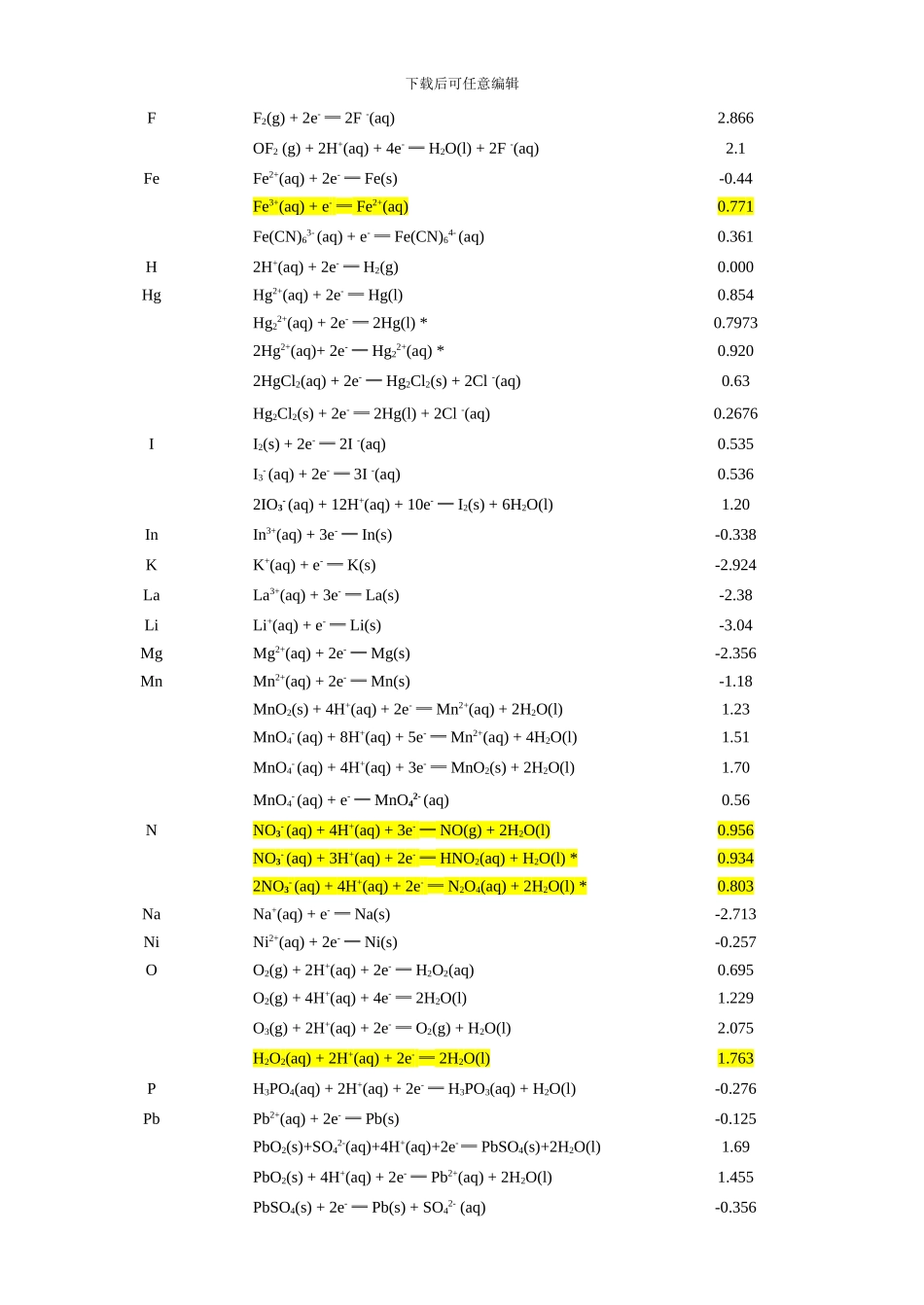
下载后可任意编辑附表 5 一些半反应的标准电极电势( 298.15K) 1. 在酸性溶液中电 极 反 应E /VAgAg+(aq) + e- ═ Ag(s)0.80Ag2+(aq) + e- ═ Ag+(aq) 1.98AgBr(s) + e- ═ Ag(s) + Br- (aq)0.071AgCl(s) + e- ═ Ag(s) + Cl- (aq)0.222AgI(s) + e- ═ Ag(s) + I- (aq)-0.152Ag2CrO4(aq) + 2e- ═ 2Ag(s) + CrO42- (aq) *0.447AlAl3+(aq) + 3e- ═ Al(s)-1.676AsHAsO2(aq) + 3H+(aq) + 3e- ═ As(s) + 2H2O(l) 0.240 H3AsO4(aq) + 2H+(aq) + 2e- ═ HAsO2(aq) + 2H2O(l) *0.560 AuAu3+(aq) + 3e- ═ Au(s)1.52Au3+(aq) + 2e- ═ Au+(aq)1.36 AuCl 4- (aq) + 3e- ═ Au(s) + 4Cl -(aq)1.002BaBa2+(aq) + 2e- ═ Ba(s)-2.92BrBr2(l) + 2e- ═ 2Br -(aq)1.0652BrO 3- (aq) + 12H+(aq) + 10e- ═ Br2(l) + 6H2O(l) 1.478 C2CO2(g) + 2H+(aq) + 2e- ═ H2C2O4(aq)-0.49CaCa2+(aq) + 2e- ═ Ca(s)-2.84CdCd2+(aq) + 2e- ═ Cd(s)-0.403ClCl2(g) + 2e- ═ 2Cl -(aq)1.358ClO3- (aq) + 6H+(aq) + 6e- ═ Cl -(aq) + 3H2O(l) 1.450 2ClO3- (aq) + 12H+(aq) + 10e- ═ Cl2(g) + 6H2O(l) *1.47 ClO4- (aq) + 2H+(aq) + 2e- ═ ClO3- (aq) + H2O(l)1.1892HClO(aq) + 2H+(aq) + 2e- ═ Cl2(g) + 2H2O(l) *1.611 CoCo2+(aq) + 2e- ═ Co(s)-0.277Co3+(aq) + e- ═ Co2+(aq) *1.92CrCr2+(aq) + 2e- ═ Cr(s)-0.90Cr3+(aq) + e- ═ Cr2+(aq)-0.424Cr2O72-(aq) + 14H+(aq) + 6e- ═ 2Cr3+(aq) + 7H2O(l)1.33CsCs+(aq) + e- ═ Cs(s)-2.923CuCu+(aq) + e- ═ Cu(s)0.52Cu2+(aq) + e- ═ Cu+(aq)0.159Cu2+(aq) + 2e- ═ Cu(s)0.34Cu2+(aq) + I -(aq) + e- ═ CuI(s) 0.86 下载后可任意编辑FF2(g) + 2e- ═ 2F -(aq)2.866OF2 (g) + 2H+(aq) + 4e- ═ H2O(l) + 2F -(aq) 2.1 FeFe2+(aq) + 2e- ═ Fe(s)-0.44Fe3+(aq) + e- ═ Fe2+(aq)0.771Fe(CN)63- (aq) + e- ═ Fe(CN)64- (aq)0.361 H2H+(aq) + 2e- ═ H2(g)0.000HgHg2+(aq) + 2e- ═ Hg(l)0.854Hg22+(aq) + 2e- ═ 2Hg(l) *0.79732Hg2+(aq)+ 2e- ═ Hg22+(aq) *0.9202HgCl2(aq) + 2e- ═ Hg2Cl2(s) + 2Cl -(aq) 0....