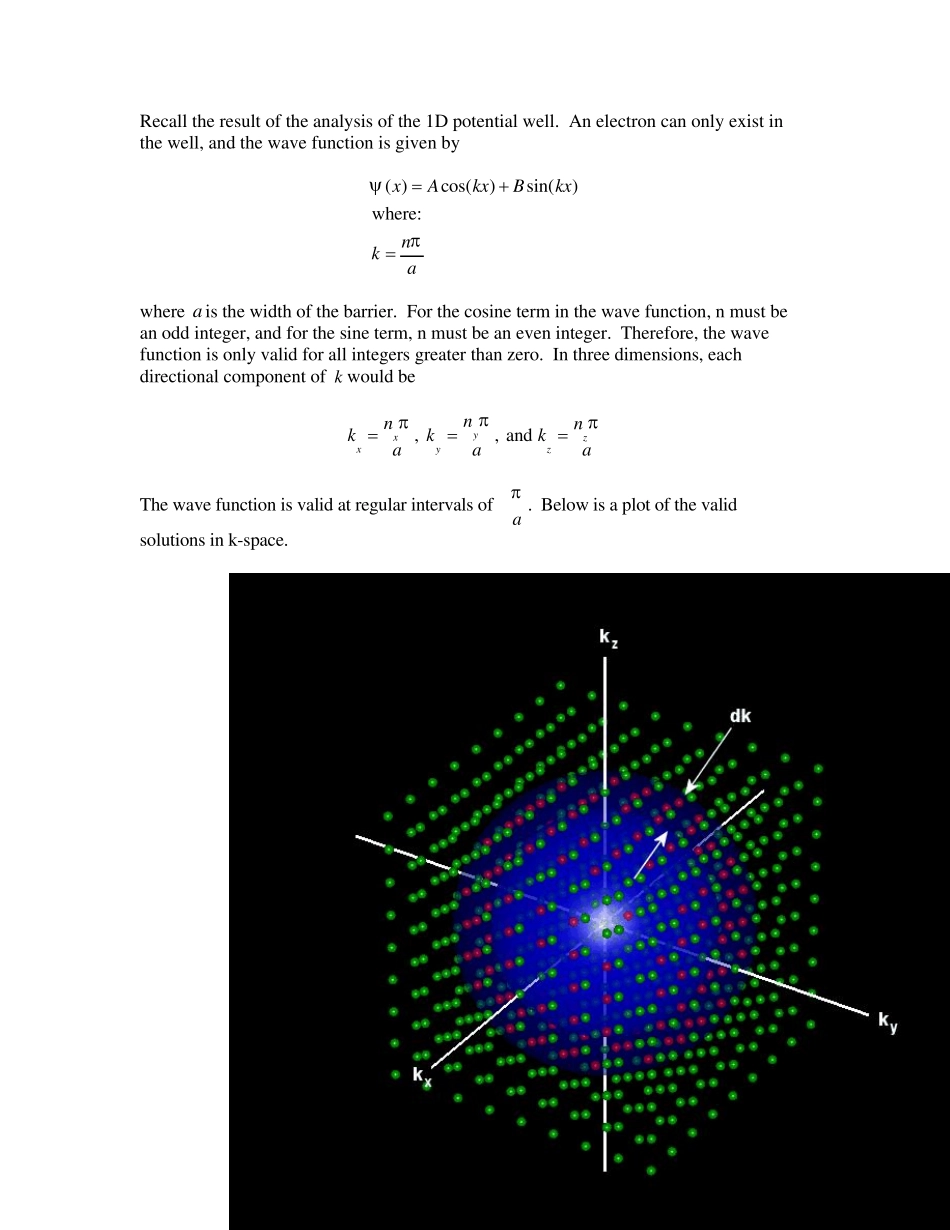
3D Density of States The density of states refers to the number of quantum states per unit energy. In other words, the density of states, denoted by ( )g E , indicates how densely packed quantum states in a particular system. So, what is the importance of the density of states? Consider the expression ( )g E dE . Integrating the density of the quantum states over a range of energy will produce a number of states. ( )( )EEN Eg E dE Thus ( )g E dE represents the number of states between and EdE . The number of quantum states is important in the determination of optical properties of a material such as a semiconductor (i.e. carbon nanotubes as well as quantum dots). From the Schrodinger equation, we know that the energy of a particle is quantized and is given by 222kEm The variable k is related to the physical quantity of momentum. A particle’s energy is 22221222m vpEmvmm Relating the previous two equations yields 22222kppEkmm The momentum is a vector which has components in the x, y, and z directions. Therefore, k must also have direction components, , and xyzkkk . Since energy is not a vector, the more accurate expression for energy is 222kEm In a 3D system, then, the total energy is given by 22222xyzEkkkm Recall the result of the analysis of the 1D potential well. An electron can only exist in the well, and the wave function is given by ( )cos()sin()where:xAkxBkxnka where a is the width of the barrier. For the cosine term in the wave function, n must be an odd integer, and for the sine term, n must be an even integer. Therefore, the wave function is only valid for all integers greater than zero. In three dimensions,...