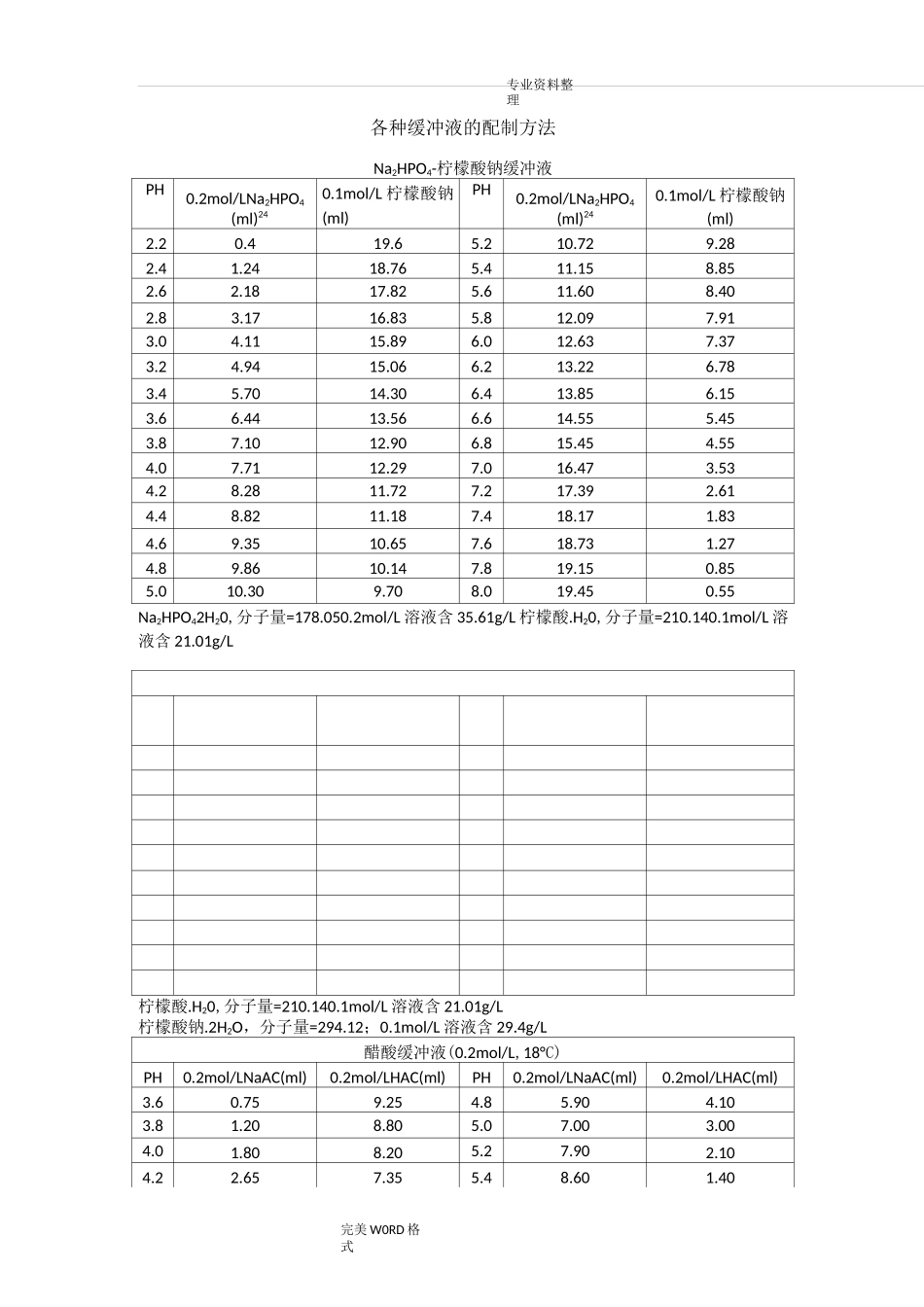
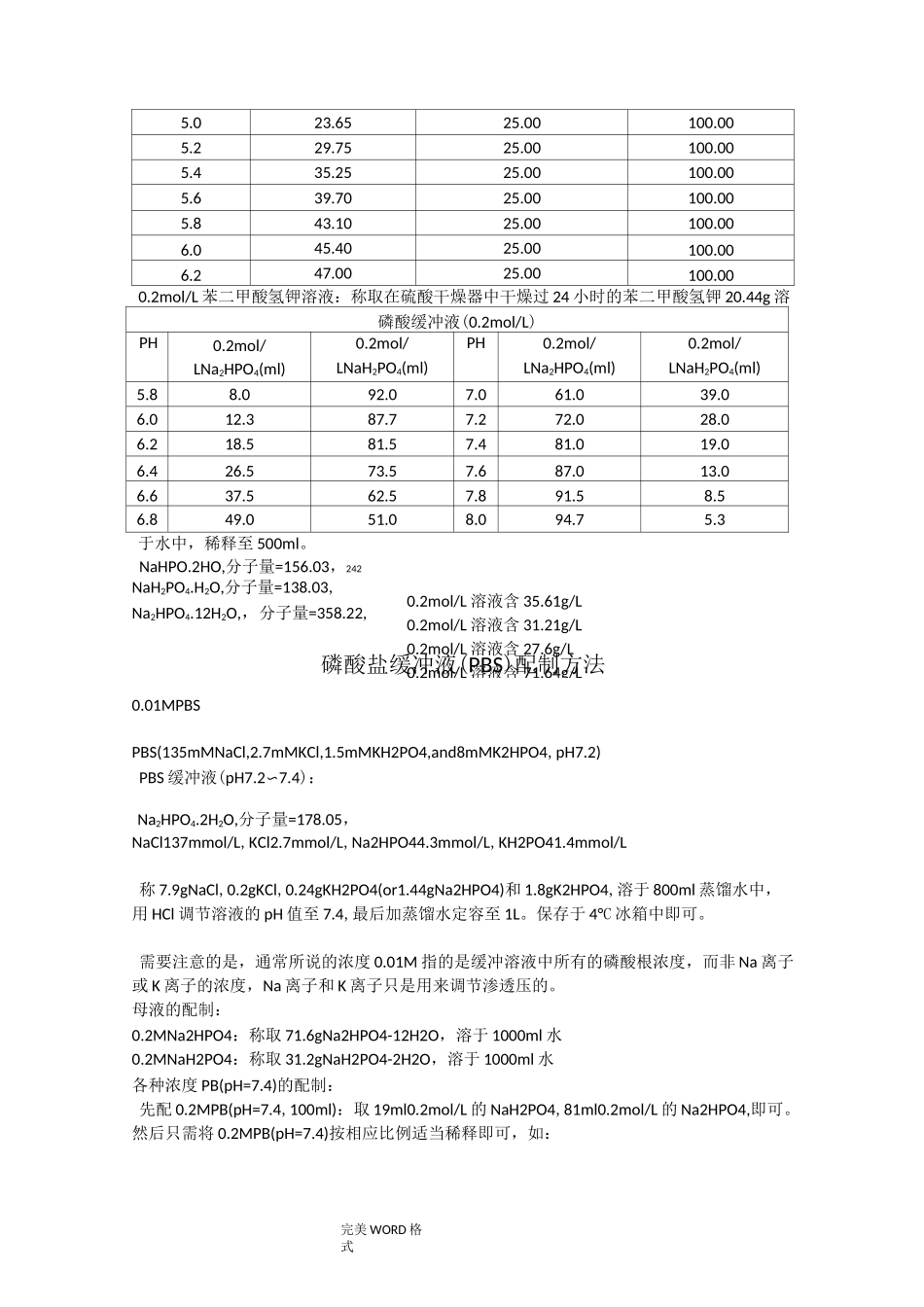
专业资料整理完美 W0RD 格式各种缓冲液的配制方法Na2HPO4-柠檬酸钠缓冲液PH0.2mol/LNa2HPO4(ml)240.1mol/L 柠檬酸钠(ml)PH0.2mol/LNa2HPO4(ml)240.1mol/L 柠檬酸钠(ml)2.20.419.65.210.729.282.41.2418.765.411.158.852.62.1817.825.611.608.402.83.1716.835.812.097.913.04.1115.896.012.637.373.24.9415.066.213.226.783.45.7014.306.413.856.153.66.4413.566.614.555.453.87.1012.906.815.454.554.07.7112.297.016.473.534.28.2811.727.217.392.614.48.8211.187.418.171.834.69.3510.657.618.731.274.89.8610.147.819.150.855.010.309.708.019.450.55Na2HPO42H20,分子量=178.050.2mol/L 溶液含 35.61g/L 柠檬酸.H20,分子量=210.140.1mol/L 溶液含 21.01g/L柠檬酸.H20,分子量=210.140.1mol/L 溶液含 21.01g/L柠檬酸钠.2H2O,分子量=294.12;0.1mol/L 溶液含 29.4g/L醋酸缓冲液(0.2mol/L,18°C)PH0.2mol/LNaAC(ml)0.2mol/LHAC(ml)PH0.2mol/LNaAC(ml)0.2mol/LHAC(ml)3.60.759.254.85.904.103.81.208.805.07.003.004.01.808.205.27.902.104.22.657.355.48.601.40完美 WORD 格式4.43.706.305.69.100.904.64.905.105.89.400.60NaAC.3H2O,分子量=136.090.2mol/L 溶液含 27.22g/L(1)醋酸盐溶液的配制:醋酸一醋酸钠缓冲液(pH3.6)取醋酸钠 5.1g,加冰醋酸 20ml,再加水稀释至 250ml,即得。醋酸一醋酸钠缓冲液(PH3.7)取无水醋酸钠 20g,加水 300ml 溶解后,加溴酚蓝指示液 1ml 及冰醋酸 60〜80ml,至溶液从蓝色转变为纯绿色,再加水稀释至 1000ml,即得。醋酸一醋酸钠缓冲液(pH3.8)取 2mol/L 醋酸钠溶液 13ml 与 2mol/L 醋酸溶液 87ml,加每 1ml 含铜 1mg 的硫酸铜溶液0.5ml,再加水稀释至 1000ml,即得。醋酸一醋酸钠缓冲液(PH4.5)取醋酸钠 18g,加冰醋酸 9.8ml,再加水稀释至 1000ml,即得。醋酸一醋酸钠缓冲液(pH4.6)取醋酸钠 5.4g,加水 50ml 使溶解,用冰醋酸调节 pH 值至 4.6,再加水稀释至 100ml,即得。醋酸一醋酸钠缓冲液(pH6.0)取醋酸钠 54.6g,加 1mol/L 醋酸溶液 20ml 溶解后,加水稀释至 500ml,即得。用醋酸和醋酸钠配制的缓冲溶液的 PH=PKa+lg[C(NaAc)/C(HAc)](在此,C(HAc)指醋酸的浓度,C(NaAc)指醋酸钠的浓度,Ka 是醋酸的解离常数=1.8*10-5(1.8 乘 10 的-5 次方),PKa=-lgKa=4.75,将 PH=5.5 代入,可得 C(NaAc)/C(HAc)=5.6 通常我们配制时会使 C(HAc)=0.1mol/L,或是 ...