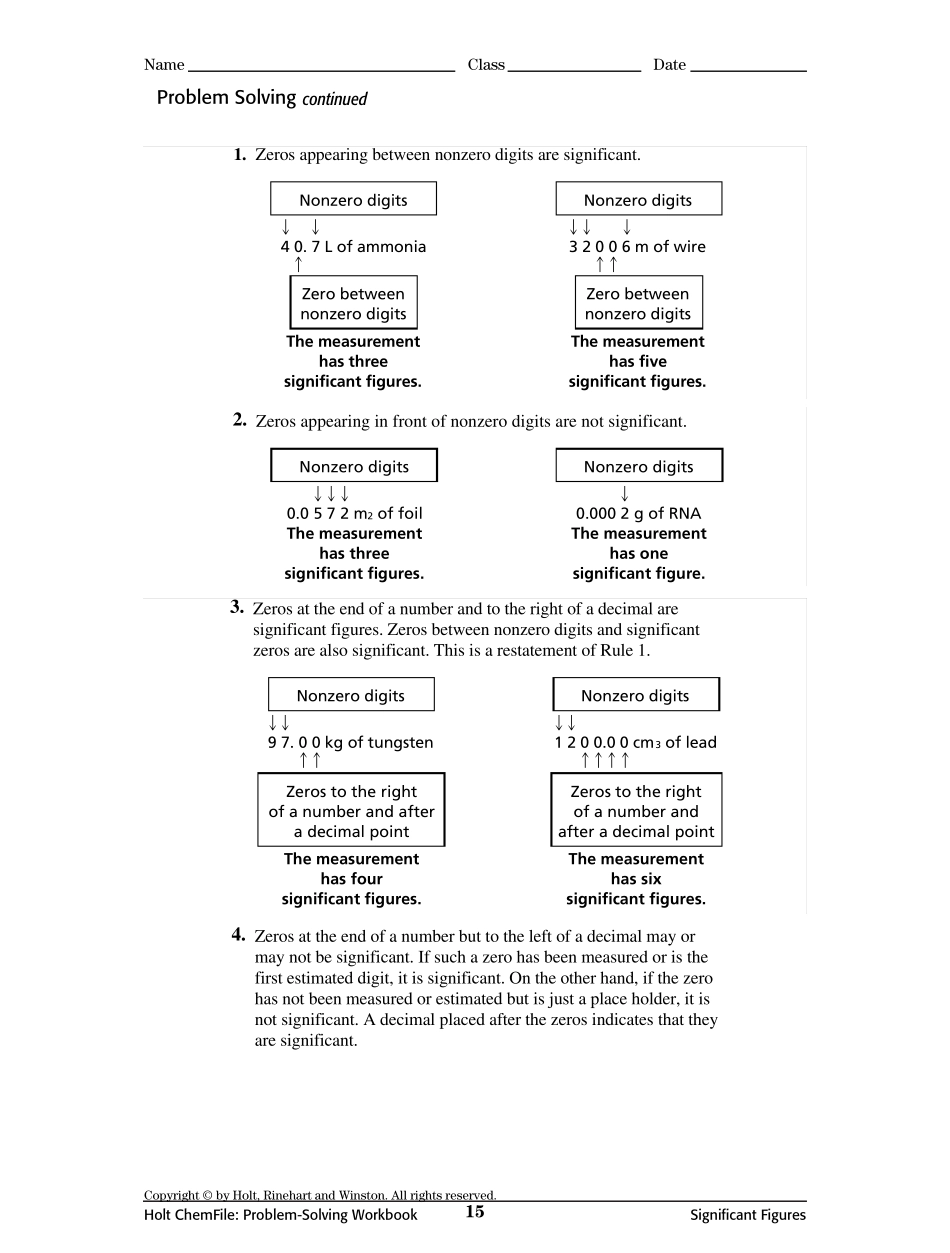
Copyright © by Holt, Rinehart and Winston. All rights reserved.Holt ChemFile: Problem-Solving Workbook14Significant FiguresSignificant Figu resA lever balance used to weigh a truckload of stone may be accurate to the near-est 100 kg, giving a reading of 15 200 kg, for instance. The measurement shouldbe written in such a way that a person looking at it will understand that it repre-sents the mass of the truck to the nearest 100 kg, that is, that the mass is some-where between 15 100 kg and 15 300 kg.Some laboratory balances are sensitive to differences of 0.001 g. Suppose youuse such a balance to weigh 0.206 g of aluminum foil. A person looking at yourdata table should be able to see that the measurement was made on a balancethat measures mass to the nearest 0.001 g. You should not state the measurementfrom the laboratory balance as 0.2060 g instead of 0.206 g because the balancewas not sensitive enough to measure 0.0001 g.To convey the accuracy of measurements, all people working in science usesignificant figures. A significant figu re is a digit that represents an actu almeasu rement. The mass of the truck was stated as 15 200 kg. The 1, 5, and 2 aresignificant figures because the balance was able to measure ten-thousands, thou-sands, and hundreds of kilograms. The truck balance was not sensitive enough tomeasure tens of kilograms or single kilograms. Therefore, the two zeros are notsignificant and the measurement has three significant figures. The mass of the foilwas correctly stated as 0.206 g. There are three decimal places in this measure-ment that are known with some certainty. Therefore, this measurement has threesignificant figures. Had the mass been stated as 0.2060 g, a fourth...