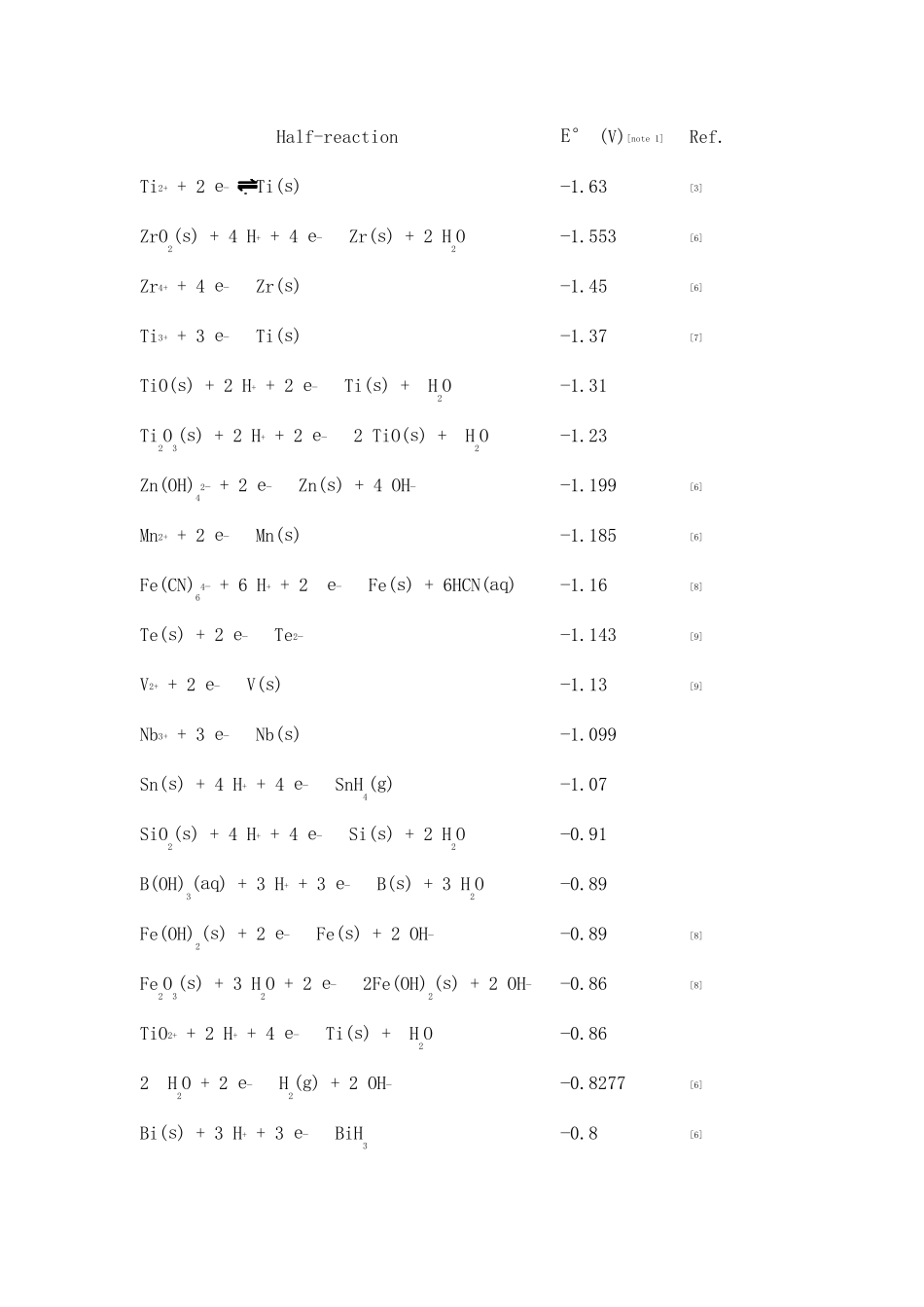
Standard electrode potential (data page) From Wikipedia, the free encyclopedia (Redirected from Table of standard electrode potentials) Jump to: navigation, search Main article: standard electrode potential The values of standard electrode potentials are given in the table below in volts relative to the standard hydrogen electrode and are for the following conditions: • A temperature of 298.15 K (25 °C); • An effective concentration of 1 mol/L for each aqueous species or a species in a mercury amalgam; • A partial pressure of 101.325 kPa (absolute) (1 atm, 1.01325 bar) for each gaseous reagent. This pressure is used because most literature data are still given for this value rather than for the current standard of 100 kPa. • An activity of unity for each pure solid, pure liquid, or for water (solvent). Legend: (s) – solid; (l) – liquid; (g) – gas; (aq) – aqueous (default for all charged species); (Hg) – amalgam. Half-reactionE° (V)[note 1] Ref. 3⁄2 N2(g) + H+ + e− HN3(aq) −3.09 [1][2]Li+ + e− Li(s) −3.0401 [2]N2(g) + 4 H2O + 2 e− 2 NH2OH(aq) + 2 OH− −3.04 [1]Cs+ + e− Cs(s) −3.026 [2]Rb+ + e− Rb(s) −2.98 [2]Half-reactionE° (V)[note 1] Ref. K+ + e− K(s) −2.931 [2]Ba2+ + 2 e− Ba(s) −2.912 [2]La(OH)3(s) + 3 e− La(s) + 3 OH− −2.90 [2]Sr2+ + 2 e− Sr(s) −2.899 [2]Ca2+ + 2 e− Ca(s) −2.868 [2]Eu2+ + 2 e− Eu(s) −2.812 [2]Ra2+ + 2 e− Ra(s) −2.8 [2]Na+ + e− Na(s) −2.71 [2][3]Sc3+ + 3 e− Sc(s) −2.077 [4]La3+ + 3 e− La(s) −2.379 [2]Y3+ + 3 e− Y(s) −2.372 [2]Mg2+ + 2 e− Mg(s) −2.372 [2]ZrO(OH)2(s) + H2O + 4 e− Zr(s) + 4 OH− −2.36 [2]Al(OH)4− + 3 e− Al(s) + 4 OH− −2.33 Al(OH)3(s) + 3 e− Al(s) + 3 OH− −2.31 H2(g) + 2 e− 2 ...