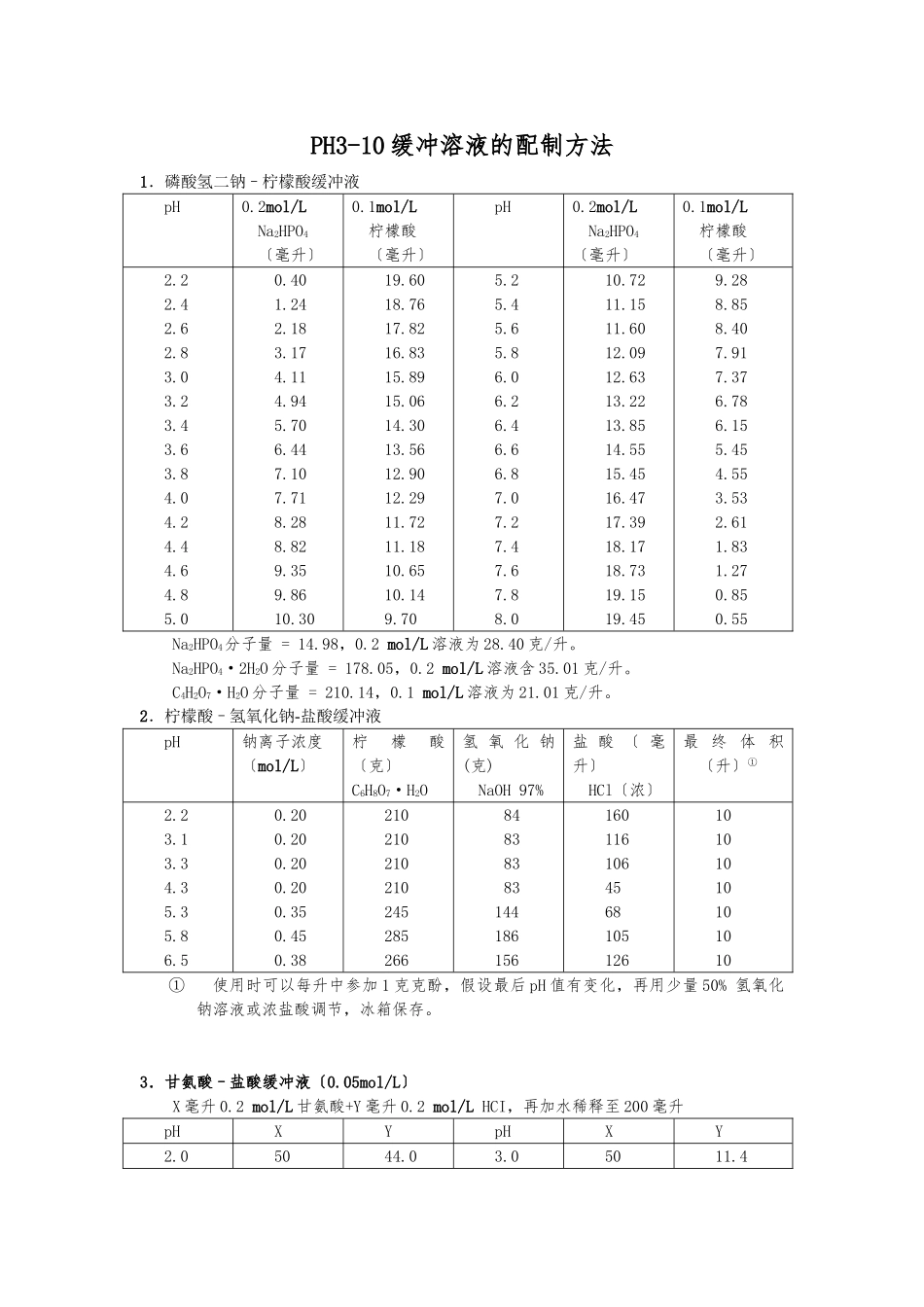
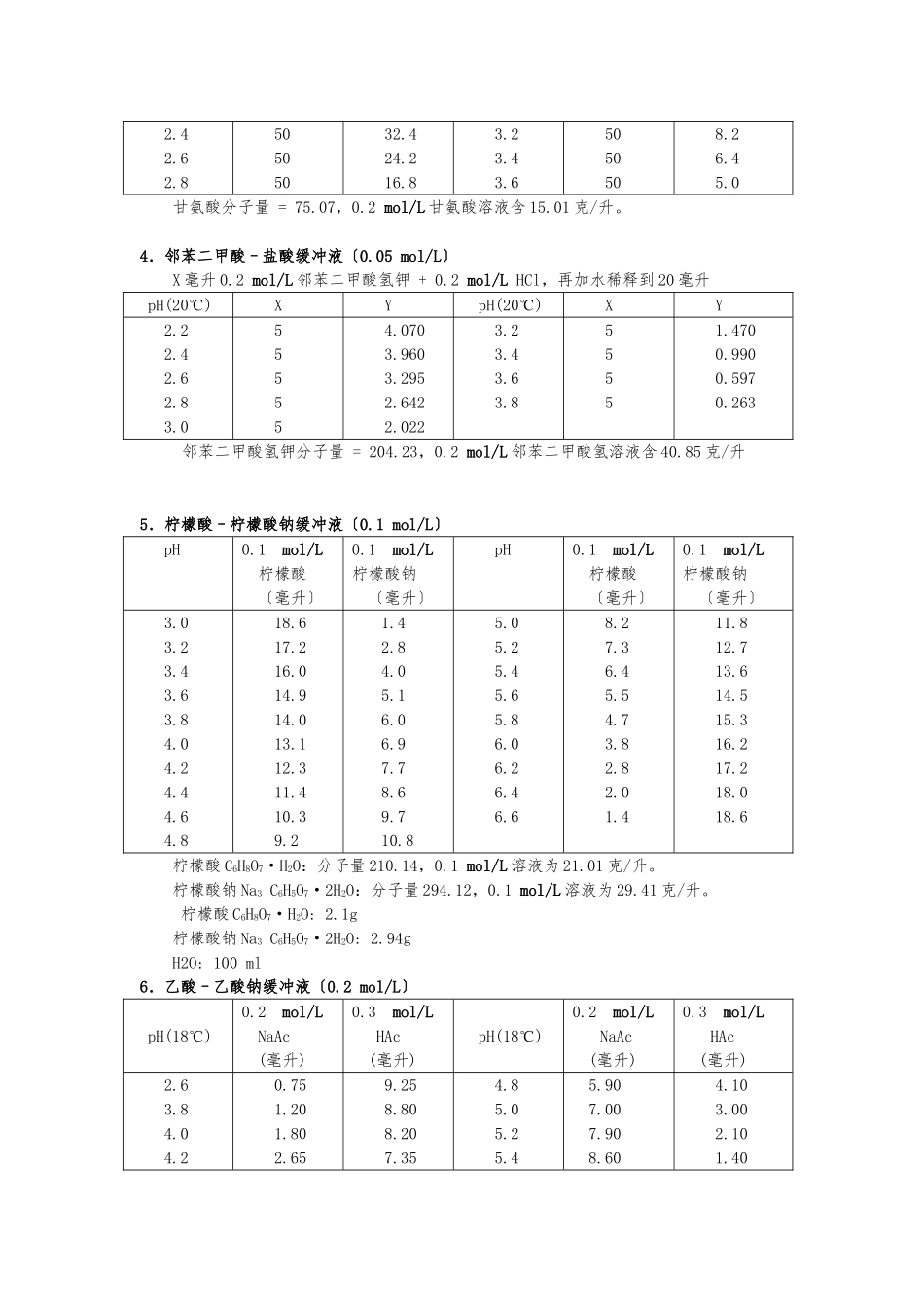
PH3-10 缓冲溶液的配制方法1.磷酸氢二钠–柠檬酸缓冲液pH0.2mol/L Na2HPO4〔毫升〕0.1mol/L柠檬酸〔毫升〕pH0.2mol/L Na2HPO4〔毫升〕0.1mol/L柠檬酸〔毫升〕2.22.42.62.83.03.23.43.63.84.04.24.44.64.85.00.401.242.183.174.114.945.706.447.107.718.288.829.359.8610.3019.6018.7617.8216.8315.8915.0614.3013.5612.9012.2911.7211.1810.6510.149.705.25.45.65.86.06.26.46.66.87.07.27.47.67.88.010.7211.1511.6012.0912.6313.2213.8514.5515.4516.4717.3918.1718.7319.1519.459.288.858.407.917.376.786.155.454.553.532.611.831.270.850.55Na2HPO4分子量 = 14.98,0.2 mol/L 溶液为 28.40 克/升。Na2HPO4·2H2O 分子量 = 178.05,0.2 mol/L 溶液含 35.01 克/升。C4H2O7·H2O 分子量 = 210.14,0.1 mol/L 溶液为 21.01 克/升。2.柠檬酸–氢氧化钠-盐酸缓冲液pH钠离子浓度〔mol/L〕柠檬酸〔克〕C6H8O7·H2O氢 氧 化 钠(克)NaOH 97%盐 酸 〔 毫升〕HCl〔浓〕最 终 体 积〔升〕①2.23.13.34.35.35.86.50.200.200.200.200.350.450.3821021021021024528526684838383144186156160116106456810512610101010101010① 使用时可以每升中参加 1 克克酚,假设最后 pH 值有变化,再用少量 50% 氢氧化钠溶液或浓盐酸调节,冰箱保存。3.甘氨酸–盐酸缓冲液〔0.05mol/L〕X 毫升 0.2 mol/L 甘氨酸+Y 毫升 0.2 mol/L HCI,再加水稀释至 200 毫升pHXYpHXY2.05044.03.05011.42.42.62.850505032.424.216.83.23.43.65050508.26.45.0甘氨酸分子量 = 75.07,0.2 mol/L 甘氨酸溶液含 15.01 克/升。 4.邻苯二甲酸–盐酸缓冲液〔0.05 mol/L〕X 毫升 0.2 mol/L 邻苯二甲酸氢钾 + 0.2 mol/L HCl,再加水稀释到 20 毫升pH(20℃)XYpH(20℃)XY2.22.42.62.83.0555554.0703.9603.2952.6422.0223.23.43.63.855551.4700.9900.5970.263邻苯二甲酸氢钾分子量 = 204.23,0.2 mol/L 邻苯二甲酸氢溶液含 40.85 克/升 5.柠檬酸–柠檬酸钠缓冲液〔0.1 mol/L〕pH0.1 mol/L柠檬酸〔毫升〕0.1 mol/L柠檬酸钠〔毫升〕pH0.1 mol/L柠檬酸〔毫升〕0.1 mol/L柠檬酸钠〔毫升〕3.03.23.43.63.84.04.24.44.64.818.617.216.014.914.013.112.311.410.39.21.42.84.05.16.06.97.78.69.710.85.05.25.45.65.86.06.26.46.68.27.36.45.54.73.82.82.01.411.812.713.614.515.316.217.218.018.6柠檬酸 C6H8O7·H2O:分子...