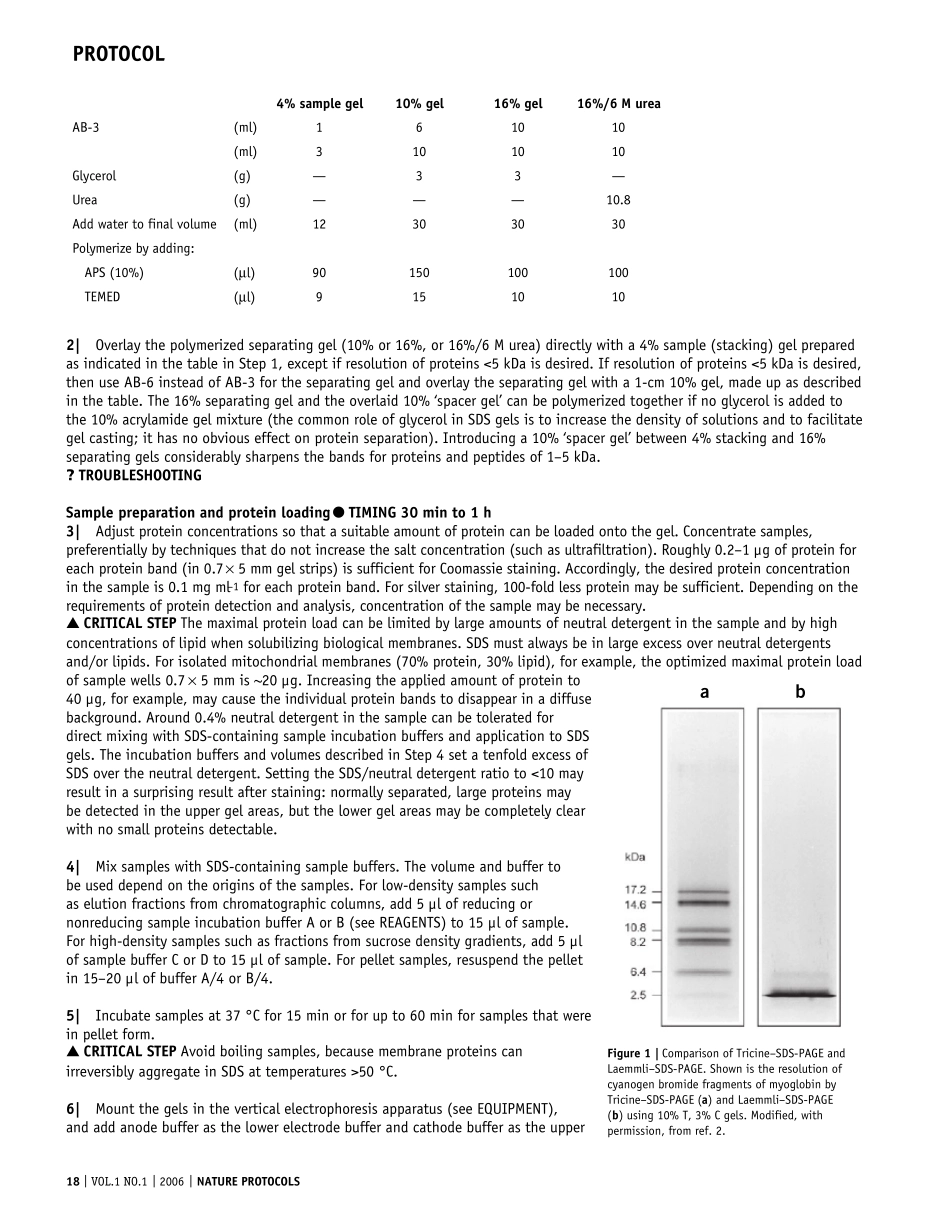
16 | VOL.1 NO.1 | 2006 | NATURE PROTOCOLSPROTOCOLTricine–SDS-PAGEHermann SchäggerMolekulare Bioenergetik, Zentrum der Biologischen Chemie, Universitätsklinikum Frankfurt, Theodor-Stern-Kai 7, Haus 26, D-60590 Frankfurt, Germany. Correspondence should be addressed to H.S. (schagger@zbc.kgu.de).Published online 12 May 2006; doi:10.1038/nprot.2006.4Tricine–SDS-PAGE is commonly used to separate proteins in the mass range 1–100 kDa. It is the preferred electrophoretic system for the resolution of proteins smaller than 30 kDa. The concentrations of acrylamide used in the gels are lower than in other electrophoretic systems. These lower concentrations facilitate electroblotting, which is particularly crucial for hydrophobic proteins. Tricine–SDS-PAGE is also used preferentially for doubled SDS-PAGE (dSDS-PAGE), a proteomic tool used to isolate extremely hydrophobic proteins for mass spectrometric identification, and it offers advantages for resolution of the second dimension after blue-native PAGE (BN-PAGE) and clear-native PAGE (CN-PAGE). Here I describe a protocol for Tricine–SDS-PAGE, which includes efficient methods for Coomassie blue or silver staining and electroblotting, thereby increasing the versatility of the approach. This protocol can be completed in 1–2 d.INTRODUCTIONGlycine–SDS-PAGE (also known as Laemmli–SDS-PAGE)1 and Tricine–SDS-PAGE2,3, based on glycine-Tris and Tricine-Tris buffer systems, respectively, are the commonly used SDS electro-phoretic techniques for separating proteins. The acrylamide gels used are often characterized by the total percentage concentration (% T) of both monomers (acrylamide and the crosslinker bisacrylamide) and the percentage concentration of the cross-linker (% C) relative to the total concent...