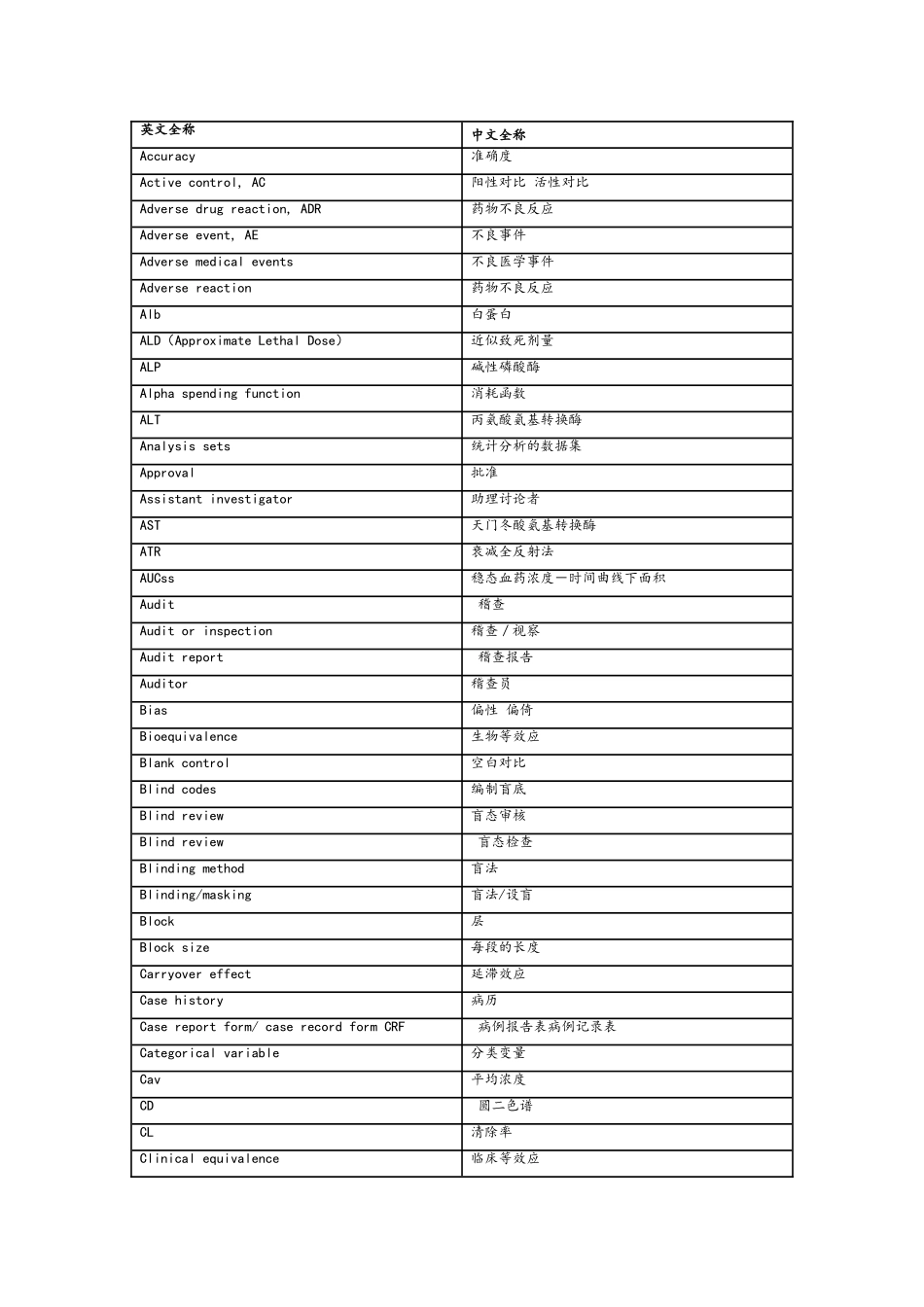
药物临床试验英文缩写缩略语 英文全称 中文全称ADE Adverse Drug Event 药物不良事件ADR Adverse Drug Reaction 药物不良反应AE Adverse Event 不良事件AI Assistant Investigator 助理讨论者BMI Body Mass Index 体质指数CI Co-investigator 合作讨论者COI Coordinating Investigator 协调讨论者CRC Clinical Research Coordinator 临床讨论协调者 CRF Case Report Form 病历报告表CRO Contract Research Organization 合同讨论组织CSA Clinical Study Application 临床讨论申请CTA Clinical Trial Application 临床试验申请CTX Clinical Trial Exemption 临床试验免责CTP Clinical Trial Protocol 临床试验方案CTR Clinical Trial Report 临床试验报告DSMB Data Safety and monitoring Board 数据安全及监控委员会EDC Electronic Data Capture 电子数据采集系统EDP Electronic Data Processing 电子数据处理系统FDA Food and Drug Administration 美国食品与药品管理局FR Final Report 总结报告GCP Good Clinical Practice 药物临床试验质量管理法律规范GLP Good Laboratory Practice 药物非临床试验质量管理法律规范GMP Good Manufacturing Practice 药品生产质量管理法律规范IB Investigator’s Brochure 讨论者手册IC Informed Consent 知情同意ICF Informed Consent Form 知情同意书ICH International Conference on Harmonization 国际协调会议IDM Independent Data Monitoring 独立数据监察IDMC Independent Data Monitoring Committee 独立数据监察委员会IEC Independent Ethics Committee 独立伦理委员会IND Investigational New Drug 新药临床讨论IRB Institutional Review Board 机构审查委员会IVD In Vitro Diagnostic 体外诊断IVRS Interactive Voice Response System 互动语音应答系统MA Marketing Approval/Authorization 上市许可证MCA Medicines Control Agency 英国药品监督局MHW Ministry of Health and Welfare 日本卫生福利部NDA New Drug Application 新药申请NEC New Drug Entity 新化学实体NIH National Institutes of Health 国家卫生讨论所(美国)PI Principal Investigator 主要讨论者PL Product License 产品许可证PMA Pre-market A...