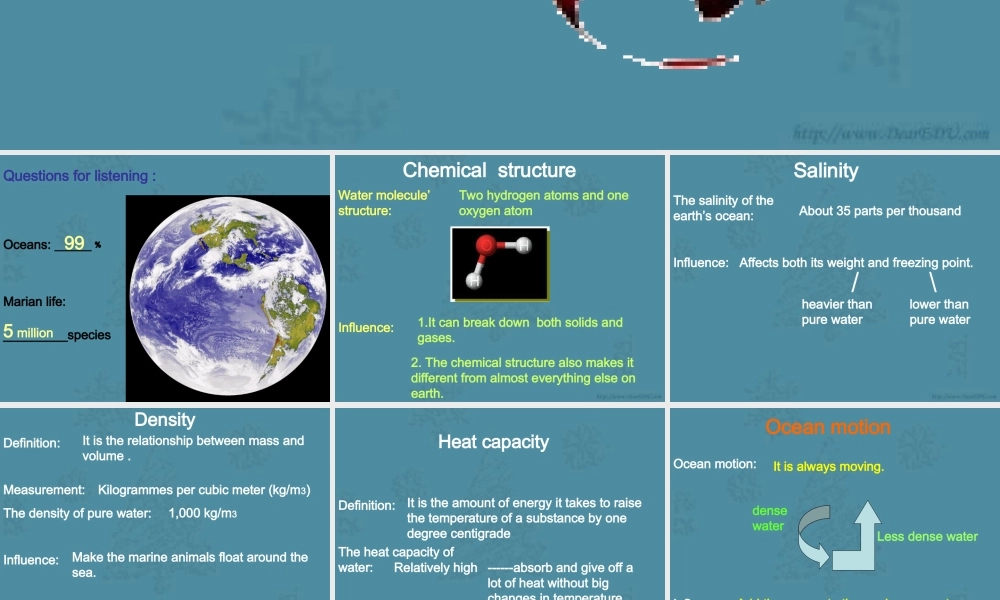
Unit 13 The Water PlanetThe Properties of WaterQuestions for listening :Oceans: ______﹪Marian life: _________species995 millionChemical structureWater molecule’ structure:Influence:Two hydrogen atoms and one oxygen atom1.It can break down both solids and gases.2. The chemical structure also makes it different from almost everything else on earth. SalinityThe salinity of the earth’s ocean:Influence:About 35 parts per thousandAffects both its weight and freezing point./heavier than pure water\lower than pure waterDensityDefinition:Measurement:The density of pure water:Influence:The change when being frozen:It is the relationship between mass and volume .Kilogrammes per cubic meter (kg/m3)1,000 kg/m3Make the marine animals float around the sea.Its density decreases.Heat capacityDefinition:The heat capacity of water:Influence:It is the amount of energy it takes to raise the temperature of a substance by one degree centigradeRelatively high ------absorb and give off a lot of heat without big changes in temperature.Make a stable environment.Ocean motionOcean motion:Influence:It is always moving.Add the energy to the marine ecosystems and moves nutrients arounddense waterLess dense water